BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2529-en.html


 , Shirin Dashtbin1
, Shirin Dashtbin1 

 , Maryam Kashanian2
, Maryam Kashanian2 

 , ُShiva Mirkalantari1
, ُShiva Mirkalantari1 

 , Nooshin Eshraghi3
, Nooshin Eshraghi3 

 , Faramarz Masjedian Jazi4
, Faramarz Masjedian Jazi4 


2- Shahid Akbarabadi Clinical Research Development Unit (ShACRDU), School of Medicine, Iran University of Medical Sciences, Tehran, Iran
3- Department of Obestrics and Gynecology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
4- Microbial Biotechnology Research Center, Iran University of Medical Sciences, Tehran, Iran & Department of Microbiology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran ,
Bacterial vaginosis (BV), also known as vaginal dysbiosis, is among the prevalent vaginal conditions linked to abnormal alterations in the vaginal microbiome (VMB) (1). BV frequently reoccurs post-treatment, with 50% of women experiencing the return of symptoms within 12 months (2, 3). Some studies indicate that it might trigger preterm labor and has been linked to the onset of pelvic inflammatory disease (PID) (3, 4). Bacterial vaginosis represents the leading cause of vaginal discharge and odor in women, impacting 29% of the female population in general. Factors contributing to the risk comprise: Black or Hispanic ethnicity, Regular douching Smoking, Multiple sexual partners, and Same-sex activity (typically affecting both individuals) (2, 5).
BV is distinguished by alterations in the composition of the vaginal flora, marked by a significant decrease in Lactobacilli and a substantial proliferation of obligate or facultative anaerobes, which were previously a minority in the vagina. These anaerobes include Gardnerella vaginalis, Atopobium vaginae, Ureaplasma urealyticum, Mycoplasma hominis, Prevotella, Peptoniphilus, Megasphaera, Mobiluncus, as well as various fastidious and uncultured bacteria, including BV-associated bacteria (BVAB-1 to 3) (1). The cause behind the proliferation of anaerobic bacteria in this context remains unidentified. It is associated with an alkaline vaginal environment resulting from an elevation in vaginal pH subsequent to the diminished protective effects of Lactobacilli (6). According to a recent prospective study, a revised conceptual model illustrating the pathogenesis of BV was delineated (7-10). The potential synergistic interaction among G. vaginalis, P. bivia, and A. vaginae was investigated (11, 12). After exposure to virulent strains of G. vaginalis through sexual contact, these strains replace the vaginal Lactobacilli and trigger the formation of a biofilm linked to bacterial vaginosis on the vaginal epithelium. (13).
Preterm birth (PTB), defined as childbirth occurring before 37 weeks of gestation, poses a significant global health concern (14). Approximately 15 million pregnancies experience PTB each year, presenting a major risk factor for neonatal mortality (15). PTB and various adverse obstetric outcomes have been linked to bacterial vaginosis (BV) in several studies (15, 16).
Studies have shown that high levels of BV-associated microbes, including A. vaginae and G. vaginalis, can be associated with PTB risk (17, 18). Other BV-associated microbes, such as Sneathia sanguinegens, Prevotella, and Mobiluncus curtsii/mulieris, are known risk factors for PTB (19). A recent multi-omic study with a large sample size showed increased levels of BV-associated microbes and a significant decrease in L. crispatus in women (15).
BV-associated microbes may contribute to infections during gestation, potentially moving into the uterus before pregnancy (13).
Given the high prevalence of BV, interventions aimed at reducing BV incidence could have a substantial impact on the occurrence of BV-associated diseases. Therefore, accurate and efficient diagnosis and treatment of BV may be crucial in preventing these diseases.
We aimed to investigate the prevalence of G. vaginalis, A. vaginae, P. bivia and L. crispatus, on pregnant women in the third trimester of pregnancy from September 2022 to April 2023 at Shahid Akbarabadi Clinical Research Development Unit (ShACRDU) by quantitative Real-Time Polymerase Chain Reaction (qPCR). Moreover, the correlation between the occurrence of PTB and the bacteria was examined.
2.1. Ethical Statement and Participant Enrollment
The study received ethical approval from the Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.FMD.REC.1401.242) and was conducted in accordance with the principles of the Helsinki Declaration. All procedures adhered to the approved guidelines, and written informed consent was obtained from all participants prior to sampling.
A total of 55 pregnant women, aged between 19 and 39 years, with no medical issues or adverse outcomes in previous pregnancies, were enrolled in the longitudinal study. Participants in the third trimester of pregnancy (between 28 and 36 weeks) were recruited at the Shahid Akbarabadi Clinical Research Development Unit from September 2022 to April 2023 and followed until delivery.
Inclusion criteria included self-reporting as Iranian, confirmation of gestational age, reproductive age (18 years or older), absence of intercurrent infections, no complications in previous or current pregnancies, no use of supplemental progesterone, the ability to provide informed consent, and willingness to participate. Exclusion criteria included intercurrent infections requiring antibiotic therapy, vaginal bleeding, recent use of antibiotics, underlying diseases such as diabetes and hypertension, kidney diseases, presence of vaginal herpes lesions, a history of uterine surgery, history of premature birth or miscarriage, and douching practices aimed at mitigating infection or PTB risks.
The research team collected foundational data, and participants were regularly followed up during antenatal visits, gathering information on maternal and clinical variables until delivery, including both term and PTB.
2.2. Sample Collection and Gram Staining Procedure
Sterile cotton-tipped swabs were used to collect vaginal discharge from the lateral vaginal wall and the posterior fornix of the vagina. These swabs were employed to apply a vaginal sample to a microscope slide, which was then subjected to Gram staining. The analysis of Gram-stained smears involved the classification of vaginal microbiota according to the criteria established by Nugent et al (20). Microscopic evaluations were conducted at up to ×1000 magnification, with scores ranging from 0 to 3 indicating normal microbiota, 4 to 6 indicating dysbiosis, and 7 to 10 indicating bacterial vaginosis (BV).
2.3. Clinical Assessment
The pregnant women underwent a clinical examination, during which a vaginal swab was obtained and assessed for BV using the Amsel criteria, proposed by Amsel et al. in 1983 (21). A diagnosis of BV is established if three out of the following four criteria are present:
1. Increased homogeneous milky vaginal discharge.
2. A pH of the secretion exceeding 4.5.
3. An amine odor observed when a 10% potassium hydroxide solution is added to a drop of vaginal secretions.
4. The presence of clue cells in wet preparations.
2.4. DNA Extraction from Swab Medium
According to the manufacturer's instructions, genomic DNA from vaginal samples was extracted using the BetaPrep Genomic DNA Extraction Kit (Nürnberg, Germany). To evaluate the quality and concentration of DNA, agarose gel electrophoresis and a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) were employed (22). The verified extracted DNAs were immediately preserved at -20°C.
2.5. Real-Time PCR (qPCR)
A quantitative real-time PCR (qPCR) was performed to assess the presence and relative quantity of microbial DNA. Primers for G. vaginalis, A. vaginae, P. bivia, and L. crispatus were used as representatives of vaginal microbial DNA. All primers were synthesized by Pishgam (Tehran, Iran), with details provided in Table 1.
Table 1. Utilized primers in the present study.
| Reference | Product size (bp) | Oligonucleotide sequence (5ʹ to 3ʹ) | Primer | Target bacteria |
| This Study | 75 | 5ʹ- TTCGCTGACCTTGATGATGC -3ʹ.. | Forward | Lactobacillus crispatus |
| This Study | 5ʹ- GGGCCATAATCCTTGCTACC -3ʹ | Reverse | ||
| This Study | 143 | 5ʹ- TGGCGTTTCAATCGCTAAGG -3ʹ | Forward | Gardnerella vaginalis |
| This Study | 5ʹ- CCAGAGATTGAGCCAACACG -3ʹ | Reverse | ||
| This Study | 129 | 5ʹ- TCAGTCATGGCCCAGAAGAC -3ʹ.. | Forward | Atopobium vaginae |
| This Study | 5ʹ- CCCTATCCGCTCCTGATACC-3ʹ | Reverse | ||
| This Study | 97 | 5ʹ- AACCCAGCGAAAGTTGGACT -3ʹ.. | Forward | Prevotella bivia |
| This Study | 5ʹ- AATCAGACGCATCCCCATCC -3ʹ.. | Reverse |
Each Real-Time PCR reaction was performed in a final volume of 20 µl, containing 0.6 μM of each primer, 10 μL of 2X Q-PCR Master Mix (SYBR, ROX) (SMOBIO, Taiwan), and 5.8 μL of sterilized ultra-pure water. The input DNA was 3 ng/reaction. The cycling conditions were as follows: initial denaturation at 95°C for 5 min; followed by 40 cycles at 95°C for 10 s, and annealing/extension at 59–61°C for 60 s. Reactions were run on the Rotor-Gene 6000 real-time PCR cycler (Qiagen Corbett, Germany). For negative controls, all ingredients of the reaction mixture were used except for template DNA.
To verify primer specificities, melting curves were generated at the end of each PCR reaction. Fluorescent data were acquired during the extension phase. After 40 cycles, a melting curve for each gene was generated by increasing the temperature from 60 to 95°C (1°C per step), while the fluorescence was measured. Samples were run in duplicates.
For the determination of the number of L. crispatus, A. vaginae, P. bivia, and G. vaginalis present in each sample, standard curves were constructed corresponding to 10^1 to 10^10 copies/ml (23). These curves were created based on the normalized copy number of the 16S rRNA gene for each species (Biosystems, 2013) and Applied Biosystems tutorials. The bacterial concentrations from each sample were calculated from the threshold cycle values (CT) obtained from the standard curves. According to previous studies (22, 23), bacterial standard strains were selected from the American Type Culture Collection (ATCC).
2.6. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 8.4.3 (GraphPad Software, San Diego, CA, USA). The Shapiro–Wilk test was used to assess the normality of the data. The Mann-Whitney U test or t-test was applied to compare two groups of continuous numerical data. Additionally, Fisher's exact test was utilized to assess the association between two categorical datasets. A p-value of less than 0.05 was considered statistically significant for the analyses.
3.1. The Characteristics of Patients with Bacterial Vaginosis and Healthy Controls
In our study, the prevalence of bacterial vaginosis was found to be 36.36%. The mean age at the time of sampling was 32.25 weeks of gestation, with a standard deviation of 2.221 weeks. And the minimum and maximum gestational ages were 28 and 36 years respectively. Our results show that there are no significant differences in delivery and maternal ages between patients with bacterial vaginosis and healthy controls (P-values > 0.05) (Table 2). However, patients with bacterial vaginosis exhibited milky vaginal discharge, positive whiff test results, Clue cells, and higher vaginal pH values compared to healthy mothers (All P-values < 0.0001) (Table 2).
3.2. The Association of Vaginal Bacterial Presence with PTB
We utilized quantitative polymerase chain reaction (qPCR) to assess the presence of the studied bacteria in the included participants. Our study indicated that the presence of G. vaginalis, L. crispatus, P. bivia, and A. vaginae was not significantly associated with PTB (All P-values > 0.05) (Table 3). In this table, concentrations above the normal limit were reported as positive, while those below were reported as negative.
3.3. The Association of Bacterial Presence with Bacterial Vaginosis Presence and Manifestations
Our results demonstrated that patients with bacterial vaginosis had lower cycle threshold (CT) values for G. vaginalis, P. bivia, and A. vaginae, and higher CT values for L. crispatus (P-values < 0.0001) (Table 4). We also examined the potential association between the CT values of each bacterium and the signs of bacterial vaginosis. It was found that individuals with a positive whiff test, milky vaginal discharge, higher vaginal pH, and Clue cells had lower CT values for G. vaginalis, P. bivia, and A. vaginae. Conversely, these individuals had higher CT values for L. crispatus (All P-values < 0.0001) (Table 4).
3.4. The Prevalence of Bacterial Vaginosis
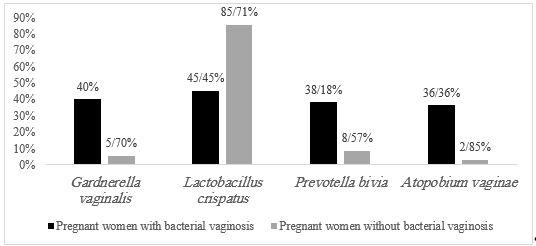
Our results showed that the prevalence of bacterial vaginosis based on the Amsel criteria is 36.36% (Table 4). The qPCR results demonstrated that the prevalence rates for G. vaginalis, L. crispatus, P. bivia, and A. vaginae were 40%, 45.45%, 38.18%, and 36.36%, respectively (Table 5 and Figure 1).
3.5. The Sensitivity, Specificity, and Predictive Values of qPCR
We also assessed the sensitivity, specificity, and positive and negative predictive values of qPCR in detecting bacterial vaginosis. Overall, the results indicated that qPCR has substantial sensitivity and specificity in detecting G. vaginalis, P. bivia, and A. vaginae. The sensitivity, specificity, and both positive and negative predictive values for qPCR in detecting these bacteria were all above 90% (Table 6).
Table 2. The characteristics of pregnant women with vaginosis and healthy pregnant women.
| Vaginosis (n=20) | Healthy (n=35) | P-value | ||
| Delivery age | 37.80 ± 1.542 | 37.80 ± 1.982 | >0.9999 | |
| Mother age | 26.35 ± 5.451 | 29.17 ± 5.451 | 0.0727 | |
| Vaginal pH | 4.375 ± 0.3193 | 3.714 ± 0.2510 | <0.0001 | |
| Vaginal discharge | Milky | 19 | 0 | <0.0001 |
| Clear | 1 | 35 | ||
| Whiff test | Present | 20 | 0 | <0.0001 |
| Absent | 0 | 35 | ||
| Clue cells | Present | 20 | 0 | <0.0001 |
| Absent | 0 | 35 | ||
| Vaginal pH | ≥ 4.5 | 17 | 0 | <0.0001 |
| < 4.5 | 3 | 35 |
Table 3. The association of vaginal bacterial with preterm delivery
| Preterm | Term | P-value | RR | 95% CI | ||
| Gardnerella vaginalis | Positive | 5 | 17 | 0.7620 | 0.8333 | 0.3225 to 2.039 |
| Negative | 9 | 24 | ||||
| Lactobacillus crispatus | Positive | 7 | 23 | 0.7619 | 0.8333 | 0.3460 to 2.021 |
| Negative | 7 | 18 | ||||
| Prevotella bivia | Positive | 4 | 17 | 0.5285 | 0.6476 | 0.2333 to 1.663 |
| Negative | 10 | 24 | ||||
| Atopobium vaginae | Positive | 4 | 15 | 0.7486 | 0.7579 | 0.2732 to 1.927 |
| Negative | 10 | 26 |
Table 4. The relationship of bacterial presence with the presence and signs of bacterial vaginosis.
| Gardnerella vaginalis (CT) | Lactobacillus crispatus (CT) | Prevotella bivia (CT) |
Atopobium vaginae (CT) | ||
| Vaginosis | Present | 19.76 ± 3.210**** | 29.21 ± 2.695**** | 24.06 ± 3.469**** | 18.79 ± 3.770**** |
| Absent | 25.87 ± 2.569**** | 23.70 ± 2.565**** | 28.00 ± 3.080**** | 23.42 ± 2.276**** | |
| Discharge | Milky | 19.64 ± 3.254**** | 29.31 ± 2.734**** | 23.98 ± 3.573**** | 18.72 ± 3.858**** |
| Clear | 25.76 ± 2.614**** | 23.80 ± 2.602**** | 27.94 ± 3.062**** | 23.33 ± 2.305**** | |
| Whiff test | Positive | 19.76 ± 3.210**** | 29.21 ± 2.695**** | 24.06 ± 3.469**** | 18.79 ± 3.770**** |
| Negative | 25.87 ± 2.569**** | 23.70 ± 2.565**** | 28.00 ± 3.080**** | 23.42 ± 2.276**** | |
| Vaginal pH | ≥ 4.5 | 20.07 ± 3.144**** | 29.24 ± 2.869**** | 24.33 ± 3.578**** | 19.11 ± 3.696**** |
| < 4.5 | 25.24 ± 3.384**** | 24.12 ± 2.895**** | 27.57 ± 3.387**** | 22.91 ± 2.290**** | |
| Clue cells | Present | 19.76 ± 3.210**** | 29.21 ± 2.695**** | 24.06 ± 3.496**** | 18.79 ± 3.770**** |
| Absent | 25.87 ± 2.569**** | 23.70 ± 2.565**** | 28.00 ± 3.080**** | 23.42 ± 2.276**** |
****: P-value < 0.0001
Table 5. The prevalence of the bacterial vaginosis.
| Bacterial vaginosis Positive based on Amsel criteria (%) | Positive based on Real-time q-PCR (%) | |
| Gardnerella vaginalis | 36.36 | 40 |
| Lactobacillus crispatus | 36.36 | 45.45 |
| Prevotella bivia | 36.36 | 38.18 |
| Atopobium vaginae | 36.36 | 36.36 |
Table 6. The sensitivity, specificity, and positive and negative predictive values of qPCR.
| Sensitivity | 95% CI | Specificity | 95% CI | Positive predictive value | 95% CI | Negative predictive value | 95% CI | |
| Gardnerella vaginalis | 100.00% | 83.16% to 100.00% | 94.29% | 80.84% to 99.30% | 90.91% | 72.25% to 97.46% | 100.00% | 89.42% to 100.00% |
| Lactobacillus crispatus | 100.00% | 83.16% to 100.00% | 85.71% | 69.74% to 95.19% | 80.00% | 63.99% to 90.01% | 100.00% | 88.43% to 100.00% |
| Prevotella bivia | 90.00% | 68.30% to 98.77% | 91.43% | 76.94% to 98.20% | 85.71% | 66.82% to 94.70% | 94.12% | 81.06% to 98.36% |
| Atopobium vaginae | 95.00% | 75.13% to 99.87% | 97.14% | 85.08% to 99.93% | 95.00% | 73.30% to 99.25% | 97.14% | 83.41% to 99.57% |
Figure 1. The percentage of bacteria abundance based on the quantitative Real time-PCR method.
Premature delivery is the primary cause of morbidity and mortality during pregnancy in most countries (24). Bacterial vaginosis has been implicated in developing PTB and subsequent complications (25). G.vaginalis, A.vaginae, and P.bivia have been introduced as the main culprits for developing bacterial vaginosis (26). Since the microbiome has a considerable variety in different geographical areas and races, we studied the impact of those bacteria PTB in Iranian mothers.
Classical diagnostic methods, such as the Amsel criteria and Nugent scoring systems, are the practical and cost-effective options for diagnosing bacterial vaginosis (27, 28). Although culture is considered the standard diagnostic approach for many bacterial infections, it is not recommended for bacterial vaginosis due to the challenges in isolation and their scarcity in normal vaginal flora (29). As an alternative diagnostic method, polymerase chain reaction (PCR) can identify the type of bacteria (30). Of interest, our results have depicted significant trends among the load of G.vaginalis, A.vaginae, P.bivia, and L. Crispatus with clinical signs and symptoms of bacterial vaginosis in our samples. The present study has shown that the prevalence of bacterial vaginosis based on Amsel's criteria is 36/36% in our 55 included cases. Consistent with this, Ruh Bakhsh et al. reported that the prevalence of bacterial vaginosis was 31% in the Gilan province of Iran in 2019 (31). The estimated prevalence of the present study was higher than the reports from Ethiopia (19.4%) and India (20.5%) and close to the reports from Kenya (37%) and Zimbabwe (32.5%) (32-35). Thus, the prevalence of bacterial vaginosis is remarkable in different geographical regions.
In this study, we demonstrated the high sensitivity and specificity of the qPCR method. Although qPCR requires specialized equipment, it proves to be more efficient and less labor-intensive than Nugent scoring, which relies on the manual assessment of Gram-stained smears. This makes qPCR a suitable option in settings with limited skilled personnel but access to basic molecular biology tools (36). While Amsel's criteria are straightforward and require minimal equipment, they exhibit lower sensitivity and specificity, increasing the risk of misdiagnosis, particularly in complicated cases. qPCR provides rapid results, which are crucial for timely treatment initiation—an essential factor in low-resource settings where diagnostic delays can worsen health outcomes (37). Furthermore, qPCR's ability to simultaneously detect multiple pathogens, including those responsible for BV, trichomoniasis, and vulvovaginal candidiasis, enables a comprehensive approach to diagnosis. This is particularly beneficial for identifying co-infections, which are common and can complicate treatment strategies (38). Overall, qPCR presents a promising tool for improving diagnostic accuracy and patient care in a variety of clinical settings.
The present study has demonstrated that mothers with bacterial vaginosis have higher levels of G. vaginalis, P. bivia, and A. vaginae bacteria, but these mothers have lower levels of L. crispatus bacteria. Our results have not identified any significant relationships between the presence of studied bacteria with PTB; this is in line with the study by Adesiji et al. (39).
The vaginal microbiome composition in our study (prevalence of Lactobacillus species) is consistent with the prevalence and microbial profile findings from the Kenyan and Zimbabwean studies, but slightly higher than from India and Ethiopia (40, 41). This discrepancy warrants further exploration into the potential influence of ethnic variations, behavioral practices (such as hygiene and dietary habits), and even diagnostic methodologies employed across these diverse populations. Roohbakhsh et al.'s 2019 study within Iran provides a valuable internal comparison, and the noted differences with other regions underscore the importance of considering the multifaceted factors that can shape the vaginal microbiome (42). Future research could benefit from standardized protocols and larger, multi-center studies to disentangle these complex interactions and provide a more comprehensive understanding of global variations in the vaginal microbiome (42).
Consistent with this, Livani et al. have reported that there is no considerable difference between the levels of G. vaginalis and A. vaginae bacteria in mothers with PTB compared to those with term delivery (43). However, Lim et al. have reported that the presence of A. vaginae is higher in patients with preterm delivery or premature rupture of membranes (44). Also, Keli et al. have shown that women with preterm delivery have low levels of Lactobacillus species and higher levels of Gardnerella, Atopobium, Megasphaera, and Streptococcus in the lower genital tract (45). Besides, Prodan-Barbulescu et al. have demonstrated that the presence of G. vaginalis is substantially associated with PTB (46). Also, Nguyen et al. have indicated that bacterial vaginosis, unlike fungal infection, increases the risk of PTB and preterm premature rupture of membranes (47).
The null association between BV-associated bacteria and PTB observed in our studies presents a paradox with other research findings. This discrepancy may arise from variability in diagnostic criteria, which can contribute to conflicting results (48). Additionally, the timing of diagnosis is crucial: BV detected before 16 weeks’ gestation shows a strong correlation with PTB, while diagnoses made later demonstrate weaker associations (49). Moreover, pathogen specificity may play a role; subclinical infections or polymicrobial interactions (50), such as those involving G. vaginalis and Mycoplasma hominis, could increase risk more than BV alone (49). For instance, one study reported a 2.1-fold increased risk of PTB when both pathogens were present (49). The host immune response is also a key factor, as BV-associated bacteria can trigger inflammatory cytokines (e.g., IL-1β, IL-6), which weaken fetal membranes (51). However, genetic or immunological variability across different populations may modulate the effects of these inflammatory responses, potentially explaining the inconsistencies observed in various studies (51).
Cultural and behavioral factors in Iran may explain these differences. For instance, lower alcohol consumption and smoking rates among Iranian women, compared to their Western counterparts, could reduce confounding behavioral risks for PTB (52).
The present study suffers from several limitations. First, we only studied bacterial vaginosis in the third trimester. Given the dynamic nature of bacterial vaginosis in pregnant women, longitudinal studies are needed to comprehensively investigate the impact of bacterial vaginosis on preterm delivery throughout all trimesters. Second, Given the cohort of only 55 participants and a prevalence of PTB at 36.36%, this study may lack the statistical power necessary to detect subtle associations, highlighting the need for more substantial sample size in future research.
Third, we utilized real-time PCR to quantify the abundance of specific bacterial groups; however, this method does not provide information about the metabolic activities of each bacterium in bacterial vaginosis. Overall, the current study provides novel insights into the bacterial vaginosis prevalence and the impact of G. vaginalis, L.crispatus, P.bivia, and A.vaginae in preterm labor of Iranian pregnant women.
In our included pregnant women, the prevalence of bacterial vaginosis is 36.36% according to the Amsel criteria. Our results have shed light on the significant trends between the load of G.vaginalis, A.vaginae, P.bivia, and L. Crispatus with clinical signs and symptoms of bacterial vaginosis in pregnant women. However, there has been no significant difference in the load of G.vaginalis, A.vaginae, P.bivia, and L. Crispatus in the lower genital tract of patients with PTB compared to those with term delivery.
The authors would like to thank the Shahid Akbarabadi Clinical Research Development Unit (ShACRDU), School Of Medicine Iran University of Medical Sciences(IUMS), Tehran, Iran for their specialists who helped collect vaginal swab samples throughout the preiod of study (IR.IUMS.FMD.REC.1401.242). All the authors have been reading and concurring with this manuscript.
Ethical Considerations
The study received ethical approval from the Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.FMD.REC.1401.242) and was conducted in accordance with the principles of the Helsinki Declaration.
Authors’ Contributions
Parisa Rahimi: Writing – original draft, Project administration, Investigation. Shirin Dashtbin: Supervision. Shiva Mirkalantari: Writing – review & editing. Maryam Kashanian: Writing – review & editing. Nooshin Eshraghi: Writing – review & editing. Faramarz Masjedian Jazi: Writing – review & editing, Resources, Project administration, Conceptualization. All authors read and approved the final manuscript.
Conflicts of Interest
This study was financially supported in Iran University of Medical Sciences (Tehran, Iran).
The authors were not utilized AI Tools.
Received: 2024/12/12 | Accepted: 2025/03/15 | ePublished: 2025/03/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |