BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2521-en.html
2- Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran ,
Honey is a complex substance produced by honeybees from the flowers nectar or secretions of living parts of plants or insects. For thousands of years, it has been valued for its nutritional and therapeutic properties (1). Honey exhibits a wide range of biological activities, including antioxidant, antimicrobial, antiviral, anti-inflammatory, anti-biofilm, anti-diabetic, and anti-cancer effects (2).
Honey is used as a natural sweetener, humectant, thickener, flavor enhancer, and antioxidant in food and beverages. It also acts as a probiotic due to the presence of lactic acid bacteria transferred by bees and as a prebiotic owing to its fructose and oligosaccharide content. Additionally, its antimicrobial activity can help reduce food spoilage (3).
Honey contains numerous enzymatic and non-enzymatic antioxidants, such as glucose oxidase, catalase, ascorbic acid, flavonoids, phenolic acids, carotenoids, organic acids, and amino acids. These compounds mitigate cellular damage caused by oxidative stress by neutralizing reactive oxygen species. Thus, consuming honey and other antioxidant-rich food may help prevent and treat oxidative stress-related diseases (3).
Honey is widely used in managing acute and chronic wound infections, as well as skin, oral, and ocular diseases (4). Approximately 65-70% of bacterial infections involve biofilm formation. These infections are chronic, and bacterial cells in the biofilm show high resistance to antibiotics (5). Honey prevents inflammation and microbial infection and plays synergistic roles with antibiotics and reduces their consumption. It also exhibits antibacterial and anti-biofilm activity against multidrug-resistant (MDR) bacteria. High osmotic pressure, acidity, hydrogen peroxide, and non-peroxide components such as phenolic compounds, methylglyoxal, and defensin are involved in the antimicrobial activity of honey. The anti-biofilm properties of honey may be attributed to its ability to disrupt the quorum sensing system, change the expression of genes related to biofilm formation, destroy membranes, and inhibit adhesion to surfaces (1). Unlike many synthetic and natural agents, the antimicrobial effect of honey is not due to a specific substance but a combination of different substances and mechanisms. Therefore, microorganisms show less resistance to it (5). Hence, honey can be a promising alternative to reduce antibiotic and preservative use and combat antibiotic resistance.
The antimicrobial, anti-biofilm, and antioxidant properties of honey vary depending on its physical and chemical composition, which are influenced by factors such as plant origin, geographical region, climate, bee species, and processing and storage conditions (6). Iran diverse climate and vegetation support the production of approximately 55 distinct honey types (7). However, limited data exist on the biological activities of Iranian honeys. Therefore, this study was designed to evaluate the antioxidant, antimicrobial, and anti-biofilm activities of honey samples of different floral origin from different regions in Iran.
2.1 Sample Collection
Iranian honey samples with different floral and geographical origins were collected from apiaries in 2024. All samples were produced by Apis mellifera carnica. The samples were transferred to the laboratory in sterile containers and stored at 18-20°C in the dark until analysis (8). Based on preliminary screening using the agar well diffusion method to assess the antimicrobial activity (data not shown), eight out of twenty samples were selected for further investigation (Table 1).
2.2 Physicochemical Analysis
The color intensity of honey was determined by converting absorbance values to the Pfund scale (8). Moisture content (gr/100 gr honey) was determined by measuring the refractive index using a refractometer (Model DR-A1-plus, ATAGO, Japan) and converting it according to Wedmore’s table (9). Electrical conductivity (EC) of 20% (w/v) honey solutions was measured using a conductivity meter (Model GLP31+, Crison, Spain), and ash content was indirectly calculated from EC values (8). The honey samples (10 gr) were dissolved in 75 ml of double-distilled water and its pH and free acidity were determined. Free acidity, expressed as milliequivalents (meq) of acid/kg of honey, was determined by titrating the sample with NaOH using phenolphthalein as an indicator (8).
2.3 Antioxidant Activity Assessment
The antioxidant activity was evaluated based on the ability to reduce the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH). Briefly, 100 µl of 0.02% (w/v) methanolic DPPH solution was mixed with 1 ml of methanol and 100 µl of honey solutions at different concentrations. The mixture was incubated at 4°C for 90 min in dark, and the absorbance was measured at 517 nm using a spectrophotometer. Ascorbic acid (10 mg/L) served as the positive control (10).
2.4 Antibacterial Activity Evaluation
The antibacterial activity of honey samples was evaluated against methicillin-resistant Staphylococcus (S.) aureus ATCC 33591 (IBRC-M 10690), Bacillus (B.) cereus ATCC 11778 (IBRC-M 10948), Escherichia (E.) coli ATCC 8739 (IBRC-M 10208), and Pseudomonas (P.) aeruginosa ATCC 10145 (IBRC-M 10828). The bacterial cultures were obtained from Microorganisms Bank, Iranian Biological Resource Center (IBRC), ACECR (Tehran, Iran).
Antibacterial activity was initially determined using the agar well diffusion method. Briefly, 5-mm diameter wells were punched into Mueller-Hinton agar plates, and bacterial suspensions adjusted to 0.5 McFarland standard were spread onto the agar surface. Subsequently, 80 µl of pure honey was added to each well. After incubation at 37°C for 24 hr, the diameter of the inhibition zone (IZ) was measured (11). Tetracycline antibiotic disk (30 µg; Padtan Teb, Iran) were used as positive control.
The minimum inhibitory concentration (MIC) of honey samples was determined using the broth dilution method in 96-well polystyrene plates. Honey solutions were prepared in the Muller-Hinton broth at concentrations ranginig from 3.125% to 50% (w/v) and sterilized by filtration through a 0.45-μm membrane filter. Aliquots of 180 µl of each dilution were transferred to the wells, followed by the addition of 20 µl of bacterial suspensions to achieve a final concentration of 10⁵ CFU/ml. The plates were incubated at 37°C for 24 hr. Wells without bacteria and wells without honey served as negative and positive controls, respectively. The MIC was defined as the lowest concentration that inhibited visible bacterial growth. To determine the minimum bactericidal concentration (MBC), samples from wells showing no growth were sub-cultured onto Nutrient agar plates. The MBC was identified as the lowest concentration that resulted in a 99.9% reduction in bacterial viability (11).
2.5 Biofilm Inhibition Assay
The anti-biofilm activity of the honey samples was evaluated against S. aureus ATCC 33591 and P. aeruginosa ATCC 10145 using the crystal violet static biofilm formation assay. Honey solutions were prepared at concentrations ranging from 3.125% to 50% (w/v) in 30% (w/v) tryptic soy broth (TSB) supplemented with 2.5 g/L of glucose. Aliquots of 180 µl of each solution were added to the wells of a flat-bottom 96-well polystyrene plate (SPL Life Science, Korea). Subsequently, 20 µl of bacterial suspensions was added to each well to achieve a final concentration of 107 CFU/ml and the plates were incubated at 37°C for 24 hr (12). Wells without bacteria and wells without honey served as negative and positive controls, respectively. After incubation, the plates were washed three times with saline solution to remove non-adherent cells. The adherent cells were fixed by adding 200 μl of methanol to each well for 15 min. Following methanol removal, the plates were stained with a 0.5% (w/v) crystal violet solution for 25 min. After washing and drying the plates, 200 μl of 33% (v/v) glacial acetic acid was added to each well to solubilize the stain. The absorbance was measured at 595 nm by a microplate reader (Model Epoch, BioTek Instruments, USA). The percentage of biofilm inhibition was calculated, and the minimum biofilm inhibitory concentration (MBIC) was defined as the lowest concentration of honey that inhibited biofilm formation by more than 90% (5).
2.6 Statistical Analysis
All experiments were performed in triplicate. Data were analyzed using Minitab 21 statistical software. Comparison of means was carried out using one-way analysis of variance (ANOVA), followed by Tukey's post-hoc test at a 95% confidence level.
3.1 Physicochemical Characteristics
Some physicochemical characteristics of the honey samples were determined using the standard methods (Tables 1 and 2). According to the international standards, the maximum permissible value for the moisture content, free acidity, electrical conductivity, and ash content are 20% (w/w), 50 mEq/kg, 800 µS/cm, and 0.6% (w/w), respectively (13). The moisture content, electrical conductivity, and ash content of all honey samples fell within the recommended ranges. The pH values of the honey samples ranged between 3.2 and 4.5, consistent with values reported for pure honey (6). However, the free acidity of Fraxinus excelsior, Astragalus-Euphorbia, and Astragalus honey samples exceeded the permissible limit. High values of free acidity may indicate microbial fermentation of sugars into organic acids. Nevertheless, factors such as type of organic acids, plant and geographical origins, and harvest season can also affect honey acidity. Notably, values higher than the permissible limit have been reported for various natural honeys, including acacia honey (6,14).
The results are presented as mean values ± standard deviation. Different letters in each column indicate significant different levels (P<0.05).
Table 1. Collection regions, botanical origins, and color of honey samples
| Honey | Botanic origin | Region (City, Province) | Pfund scale (mm) | Color |
| H1 | Ziziphus spina-christi (Sidr) | Gotvand, Khuzestan | 78 | Pale amber |
| H3 | Multifloral | Kamyaran, Kurdistan | 70 | Pale amber |
| H5 | Glycyrrhiza glabra (Licorice) | Shiraz, Fars | 38 | Very pale amber |
| H6 | Citrus | Jahrom, Fars | 48 | Very pale amber |
| H8 | Astragalus (Milkveteh) | Aligudarz, Lorestan | 44 | Very pale amber |
| H15 | Quercus brantii (Oak) | Dezful, Khuzestan | 47 | Very pale amber |
| H18 | Fraxinus excelsior (Ash) | Hamedan, Hamedan | 73 | Pale amber |
| H19 | Astragalus-Euphorbia (Milkveteh-Spurge) | Hamedan, Hamedan | 109 | Amber |
Table 2. Physicochemical characterization of the honey samples
| Honey | Moisture content (%) | EC (µS/cm) | Ash content (%) | pH | Acidity (meq/kg) |
| H1 | 14.0±0.0a | 527±7a | 0.22±0.01 a | 4.4±0.2 a | 32.8±0.8 f |
| H3 | 14.1±0.0 a | 316±6 d | 0.10±0.01 c | 3.4±0.0 b | 39.7±0.8 ef |
| H5 | 14.1±0.0 a | 213±4 e | 0.04±0.0 d | 3.6±0.0 b | 32.8±0.8 f |
| H6 | 14.0±0.0a | 346±6 c | 0.12±0.01 c | 3.5±0.0 b | 50.6±1.6 d |
| H8 | 14.1±0.0 a | 226±4 e | 0.04±0.01 d | 3.1±0.0 c | 59.8±1.6 c |
| H15 | 13.9±0.0 a | 223±4 e | 0.04±0.0 d | 3.4±0.0 bc | 44.3±0.8 de |
| H18 | 14.0±0.0a | 311±6 d | 0.10±0.01 c | 3.4±0.1 bc | 101.2±4.9 a |
| H19 | 14.0±0.0a | 433±7 b | 0.16±0.01 b | 3.5±0.0 b | 69.0±1.6 b |
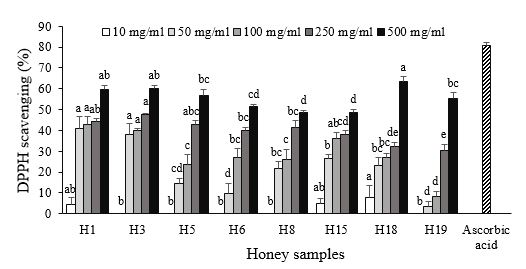
3.2 Honey Samples Showed Antioxidant Activity at Higher Concentrations
The antioxidant activity of honey plays a significant role in maintaining food quality, promoting wound healing, and preventing various diseases by neutralizing free radicals. This activity is primarily attributed to the quantity and type of phenolic compounds derived from plant source (3). In this study, DPPH radical scavenging activity of the honey samples was moderate compared to those previously reported for different types of honey. At the highest concentration tested (500 mg/ml), the antioxidant activity ranged from 48.4% to 63.6%, showing relatively close values among the samples. However, at concentration of 50 mg/l, Ziziphus spina-christi and multifloral honeys exhibited significantly higher antioxidant activity compared to other samples (Figure 1). Shakoori et al. reported that DPPH scavenging activity for 57 types of Iranian honey samples ranged from 19 to 98%, and it was affected by the botanical and geographical origins of the honey samples (7). Zarei et al. reported the antioxidant activity of five Iranian honey samples within the range of 19 to 65%, with Astragalus honey showing 19% activity and Ziziphus and multifloral honeys exhibiting 45% (8). In the present study, the antioxidant activity was found to be 48% for Astragalus honey and 60% for Ziziphus and multifloral honey samples.
Figure 1. Antioxidant activity of honey samples at various concentrations. Different letters above the columns indicate significant differences (P<0.05) among the honey samples for the related concentration. Honey samples codes: H1 (Ziziphus spina-christi), H3 (Multifloral), H5 (Glycyrrhiza glabra), H6 (Citrus), H8 (Astragalus), H15 (Quercus brantii), H18 (Fraxinus excelsior), H19 (Astragalus-Euphorbia).
3.3 Honey Samples Exhibited Diverse Antibacterial Activity
Based on the results, the antibacterial effects varied among different honey samples (Table 3). The MIC and MBC values of the honey samples ranged from ≤3.125% to >50% (w/v). When the MBC/MIC ratio is ≤4, the antimicrobial agent is considered bactericidal, otherwise it is bacteriostatic (15). Accordingly, the Citrus and multifloral honeys exhibited a bacteriostatic effect against E. coli, while a bactericidal effect was observed in other cases. Overall, the honey samples showed stronger inhibitory effects against E. coli and S. aureus and weaker effects against B. cereus and P. aeruginosa. The MIC values for various types of honey against different bacteria have been reported from 1.56% to 100% (w/v) (1). Mahmoodi-Khaledi et al. reported that MIC and MBC values of 53 Iranian honeys ranged from 3.12% to over 50% (w/v), and consistent with our results, E. coli and S. aureus were more sensitive to honey than P. aeruginosa (16). While most studies indicate that Gram-positive bacteria, particularly S. aureus, are more sensitive to honey, some studies have found greater sensitivity in Gram-negative bacteria (1). In this study, the highest antibacterial activity was observed in the F. excelsior and Astragalus-Euphorbia honeys, while the lowest activity was observed in the G. glabra honey. Since the bee breed was the same in all honeys, the observed differences in antibacterial activity may be attributed to the factors such as floral origin, weather conditions, and processing methods (6). Although the antibacterial activity of Ziziphus, G. glabra, Citrus, Astragalus, Q. brantii, Euphorbia, and multifloral honeys has been reported in various studies around the world, no previous reports exist on the antibacterial activity of F. excelsior and Astragalus-Euphorbia honeys.
Manuka honey is one of the well-known types of honeys widely used in the pharmaceutical industry for the treatment of various diseases and is recognized as a medical-grade honey. Recent research on antibacterial properties of different honey types has revealed that some exhibit similar or even higher antibacterial activity compared to Manuka honey, suggesting their potential for the similar applications (1). The MIC values of Manuka-type honeys against various strains of S. aureus, E. coli, and P. aeruginosa have been reported in the ranges of 1.56-12.5%, 3.7-25%, and 9.5-25% (w/v), respectively (1,16). In the present study, the MIC values of Q. brantii, multifloral, and Citrus honeys against E. coli were ≤3.125% (w/v), indicating higher antibacterial activity than that of Manuka honey. Additionally, the MIC values of three honey samples against E. coli, six honey samples against S. aureus, and five honey samples against P. aeruginosa (Table 3) were in the ranges reported for the medical-grade Manuka honeys, highlighting their potential for the therapeutic use.
The average inhibition zone (IZ) is in mm and the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values are in percentage (w/v).
Table 3. Antibacterial activity of the honey samples
| Honey | S. aureus | B. cereus | E. coli | P. aeruginosa | ||||||||
| IZ | MIC | MBC | IZ | MIC | MBC | IZ | MIC | MBC | IZ | MIC | MBC | |
| H1 | 21 | 12.5 | 12.5 | 12.5 | 25 | 25 | 15 | 6.25 | 12.5 | 10.5 | 25 | 25 |
| H3 | 12 | 25 | 25 | 6 | 50 | 50 | 18 | ≥3.125 | 12.5 | - | 50 | 50 |
| H5 | - | 50 | >50 | - | 50 | 50 | 11.5 | 25 | 50 | 8 | 50 | >50 |
| H6 | 19 | 12.5 | 25 | 13 | 25 | 50 | 20 | ≥3.125 | 25 | 10.5 | 50 | 50 |
| H8 | 25 | 6.25 | 6.25 | 13 | 25 | 25 | 11 | 12.5 | 12.5 | 9 | 25 | 25 |
| H15 | 20 | 12.5 | 12.5 | 10 | 25 | 25 | 17.5 | ≥3.125 | ≥3.125 | 8 | 25 | 25 |
| H18 | 25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 11 | 12.5 | 12.5 | 10 | 12.5 | 12.5 |
| H19 | 23 | 6.25 | 12.5 | 11.5 | 12.5 | 12.5 | 9 | 12.5 | 12.5 | 8.5 | 12.5 | 12.5 |
| Tetracycline | 9 | 29 | 19 | 11.5 | ||||||||
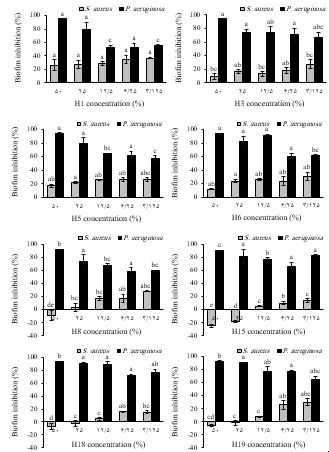
3.4 Anti-biofilm Activity Was Observed in Honey Samples
P. aeruginosa and methicillin-resistant S. aureus play a critical role in the drug-resistant hospital infections and biofilms. Biofilms produced by these bacteria are also commonly found on the surface of chronic wounds (5). The anti-biofilm activity of Iranian honeys against these bacteria is illustrated in Figure 2.
Figure 2. Effect of honey samples on biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa. Different letters above the columns indicate significant difference (P<0.05) among the honey samples for the related concentrations. Honey sample codes: H1 (Ziziphus spina-christi), H3 (Multifloral), H5 (Glycyrrhiza glabra), H6 (Citrus), H8 (Astragalus), H15 (Quercus brantii), H18 (Fraxinus excelsior), H19 (Astragalus-Euphorbia).
The honey samples significantly inhibited biofilm formation by P. aeruginosa. The MBIC values against P. aeruginosa were 25% (w/v) for F. excelsior and Astragalus-Euphorbia honeys and 50% (w/v) for other honeys. Generally, as the concentration of honey decreased, a slight reduction in anti-biofilm activity against P. aeruginosa was observed. Howevere, even at the lowest concentration tested (3.125% w/v), the biofilm mass was reduced by 56-83%. In contrast, none of the honey samples completely inhibited biofilm formation by S. aureus. The highest inhibitory effect (37%) was achieved by Z. spina-christi honey at 3.125% (w/v) concentration. Notably, no inhibition was observed with 25% and 50% (w/v) concentrations of Astragalus, F. excelsior, and Astragalus-Euphorbia honeys, and Q. brantii honey even the biofilm formation increased slightly in these concentrations.
The MBIC value of different honeys against P. aeruginosa has been reported to range from 2% to 50% (w/v), likely due variations in honey types and bacterial strains studied (17). Mahmoudi Khalidi et al. reported that the MBIC values of nine Iranian honeys (without mentioning plant origin) against P. aeruginosa were in the range of 3.12-25% (w/v) and against S. aureus were in the range of 6.25-25% (w/v) (16). In other studies, the MBIC values of Manuka honeys against P. aeruginosa were reported to be 8-32% (w/v) and against S. aureus to be 8-16% (w/v) (17,18). Ghotaslou et al. reported 62%, 64%, and 69% inhibition of P. aeruginosa biofilm formation using 12.5% (w/v) Chamaemelum nobile, 12.5% (w/v) Medicago sativa and 3.125% (w/v) Stachys inflata honeys, respectively (19). In another study, the MBIC value of most Manuka, Eucalyptus, and multifloral honeys against P. aeruginosa and S. aureus was 30% (w/v), although one Eucalyptus honey increased biofilm formation by S. aureus at this concentration (20). Lu et al. suggested that the inhibitory effect of Manuka honey on P. aeruginosa biofilm formation can be primarily attributed to its high sugar content, which may induce osmotic stress, alter carbon metabolism, and disrupt quorum sensing (17). The concentration-dependent inhibitory effect of Iranian honeys against P. aeruginosa biofilm formation may also be explained by this mechanism. However, other antimicrobial components in honey could contribute to this activity. In contrast, such a relationship was not observed for S. aureus. At ≥25% (w/v) concentrations, Q. brantii honey even slightly increased biofilm formation, possibly due to the stimulatory effect of sugars, which serve as energy sources and building blocks for biofilm formation. Additionally, hydrogen peroxide may stimulate biofilm formation (20). Various biofilm-specific mechanisms, as well as mechanisms dependent on antimicrobial activity, have been proposed to explain the anti-biofilm effects of honey (20).
This study highlights the beneficial properties of certain Iranian honeys, including antioxidant, antibacterial, and anti-biofilm activities. To the best of our knowledge, this is the first report on anti-biofilm effects of Iranian G. glabra, Citrus, Astragalus, F. excelsior, and Astragalus-Euphorbia honeys. This activity was particularly against P. aeruginosa. Honeys with different botanical origins showed different physicochemical and biological properties. Further reseach, including the identification of active compounds, elucidation of their mechanisms of action, and clinical trials, could reveal the potential applications of these honeys in medicine, pharmaceuticals, food, and cosmetics. Such efforts may also support the commercial production of honey varieties tailored for the specific applications.
The authors acknowledge the laboratory staff of Science and Research Branch, Islamic Azad University for providing the facilities.
Ethical Considerations
No human or animal subjects were involved in this study, and no ethical approval was not applicable.
Authors’ Contributions
Amir Hosseini-nejad contributed to the conception and design, data acquisition, analysis, and interpretation, drafting the manuscript. Nayyereh Alimadadi contributed to the conception and design, revising for important intellectual content and final approval of the published version. All authors have read and approved the final version of the manuscript. The final manuscript was read and approved by all of the authors.
Conflicts of Interest
This research project was conducted without any financial support from external sources.
The authors were not utilized AI Tools.
Received: 2024/11/21 | Accepted: 2025/02/20 | ePublished: 2025/03/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |