BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2502-en.html
2- Department of Microbiology, Research Center of Health Reference Laboratory, Ministry of Health and Medical Education, Tehran, Iran
3- Department of Microbiology, School of Biological Sciences, North Tehran Branch, Islamic Azad University, Tehran, Iran ,
Enterococci are a group of bacteria that are commonly found in human gut and other environmental niches. While they are generally considered to be harmless, certain strains have developed resistance to multiple antibiotics, posing a significant threat to public health (1, 2). Among these strains are Enterococcus (E.) faecium and E. faecalis, which have been recognized as important nosocomial pathogens and frequently involved in serious infections in hospitalized patients, particularly in immunocompromised individuals. One of the most concerning antibiotic resistance traits in Enterococci is their resistance to vancomycin, which is often considered the last line of defense against severe infections caused by Gram-positive bacteria (3, 4).
Vancomycin resistance in E. faecium and E. faecalis presents a significant challenge in clinical settings, requiring a comprehensive understanding of the mechanisms involved to effectively combat this threat (5). Enterococci are commensal organisms in human gastrointestinal tract, but they have also emerged as leading causes of healthcare-associated infections, particularly due to their ability to acquire resistance to multiple antibiotics, including vancomycin. The acquisition and dissemination of vancomycin resistance genes among enterococcal populations have contributed to the reduced efficacy of this critical antibiotic, posing a serious public health concern (6, 7). Understanding the molecular mechanisms underpinning vancomycin resistance in these species is essential for guiding therapeutic strategies and combating the spread of resistance (8).
Molecular studies have revealed that vancomycin resistance in E. faecium and E. faecalis is often mediated by acquisition of genetic elements such as vancomycin resistance genes, including vanA, vanB, and vanC clusters. These genes encode proteins that modify peptidoglycan cell wall structure, thereby reducing the binding affinity of vancomycin and conferring resistance to the antibiotic (9, 10). The emergence of these resistant strains limits the therapeutic options available for treating enterococcal infections, particularly those caused by multidrug-resistant (MDR) organisms. In addition, the spread of these resistant strains can lead to outbreaks in healthcare settings, further complicating infection control efforts (11, 12).
The study of gene expression in VRE is increasingly necessary due to the rising prevalence of antibiotic-resistant infections, which pose significant challenges to healthcare systems worldwide. The emergence of healthcare-associated infections, including those caused by VRE, emphasizes the importance of understanding the molecular mechanisms behind this resistance, particularly through the expression of specific genes such as vanA and vanB. The unique epidemiology of VRE, influenced by factors such as overuse and misuse of antibiotics, inadequate infection control practices, and variability in healthcare infrastructure, highlights the necessity for localized research. By characterizing gene expression patterns in VRE isolates, researchers can better understand resistance mechanisms, facilitating the design of targeted interventions and antimicrobial stewardship programs that reflect local realities.
Additionally, VRE poses a significant threat to vulnerable populations, such as immunocompromised and patients in intensive care units, which are prevalent due to various health issues. Conducting gene expression studies specifically focused on VRE can contribute to enhancing infection control measures, ultimately reducing the incidence and spread of these MDR organisms within healthcare settings.
Understanding the molecular mechanisms behind vancomycin resistance in E. faecium and E. faecalis is essential for developing effective treatment strategies. This research is guided by the hypothesis that specific genetic and biochemical factors contribute to vancomycin resistance in these bacterial strains. The key questions addressed in this study include: What are the underlying genetic determinants of vancomycin resistance in E. faecium and E. faecalis? How does the expression of vanA gene influence this resistance?
The primary aim of this study was to synthesize current knowledge regarding molecular mechanisms of vancomycin resistance in these two species, with a particular emphasis on the role of vanA gene and its expression patterns. Through extensive examination of existing research, this study aimed to clarify the genetic and biochemical basis of resistance, ultimately contributing to improved treatment strategies for infections caused by these resistant bacteria.
Materials and Methods
A total of 120 clinical Enterococcus isolates were randomly collected from various departments of Bahonar Hospital (Tehran, Iran) between 2021 and 2023. The samples were derived from various sources, including urine (n=76, 63.3%), tracheal (n=23, 19.2%), blood (n=14, 11.6%), wound (n=7, 5.8%). Identification of Enterococcus genus was carried out using standard microbiological techniques. Phenotypic detection of E. faecalis and E. faecium involves a series of systematic steps aimed at identifying these bacteria based on their morphological and biochemical characteristics. Initially, clinical specimens were inoculated onto nutrient media such as blood agar, which promote the growth of Enterococcus species. After incubation at 35-37°C for 24-48 hr, the resulting colonies were examined for their distinctive morphology; Enterococcus typically forms small, grayish colonies with a rough texture. The identification process continued with catalase test, where a negative result -indicated by the absence of bubbles upon addition of hydrogen peroxide- confirms identity of the isolate. Subsequently, a bile esculin hydrolysis test was performed, where a color change in the medium indicates the organism ability to hydrolyze esculin in the presence of bile salts. Sugar fermentation tests were also employed, evaluating the organism ability to ferment various carbohydrates, with E. faecalis often exhibiting a broader fermentation profile compared to E. faecium. To further support identification, a sodium chloride tolerance test was conducted, where successful growth in a high-salt medium confirmed the isolate resilience typical of Enterococcus species. Following this, PCR analysis targeting D-alanine-D-alanine ligases specific for E. faecalis (ddl E. faecalis) and E. faecium (ddl E. faecium) was conducted to verify the phenotypic speciation. PCR for identification of E. faecalis and E. faecium was executed with specific primers designed for each species. For E. faecalis, forward primer was 5’-ATCAAGTACAGTTAGTCT-3’ and the reverse primer was 5’-ACGATTCAAAGCTAACTG-3’, resulting in a 941 bp PCR product. For E. faecium, forward primer was 5’-TAGAGACATTGAATATGCC-3’ and reverse primer was 5’-TCGAATGTGCTACAATC-3’, yielding a 550 bp PCR product. A specificity check was performed using Primer BLAST in NCBI database with the target template and both primers provided. The amplification reactions were conducted using Eppendorf Thermal Cycler (Eppendorf, Germany) in 0.5 ml MicroAmp reaction tubes. Each reaction mixture was prepared to include 12.5 µl master-mix red, sourced from Ampliqon, Denmark. The final reaction volume of 25 µl contained 50 pmol of each primer, ensuring optimal conditions for the specific amplification of the target DNA regions. The reaction conditions for ddl-PCR were as follows: an initial denaturation at 94°C for 1 min. This was followed by 30 cycles consisting of denaturation at 90°C for 30 sec, annealing at 54°C for 30 sec, and polymerization at 72°C for 60 sec. Finally, an extension step was included at 72°C for 8 min. This methodology allowed for the reliable detection of E. faecalis and E. faecium in various samples through the precise amplification of their respective genetic material (13).
Antimicrobial Susceptibility Testing
The susceptibility to vancomycin (30 μg), erythromycin (15 μg), ampicillin (10 μg), penicillin (10 U) and ciprofloxacin (5 μg) was assessed using disk diffusion method in accordance with the guidelines set by the Clinical and Laboratory Standards Institute (CLSI). The susceptibility testing disks were produced by HIMEDIA, India. The diameters of the inhibition zones were measured in millimeters (mm) and interpreted as susceptible, intermediate, or resistant. For the purposes of this study, intermediate results were considered as resistant. The E-test method was utilized to confirm the presence of vancomycin-resistant isolates. The minimum inhibitory concentration (MIC) values obtained through E-test for vancomycin susceptibility in Enterococci were interpreted based on the CLSI breakpoints. Additionally, a reference strain, E. faecalis 29,212, was employed as control strain during testing process (14).
Study of the Gene Responsible for Vancomycin Resistance
DNA extraction was performed on all presumptive Enterococcus isolates using a commercial DNA extraction kit (High Pure PCR template preparation kit, ROCHE, Germany). To determine the presence of specific genetic markers for vancomycin-resistant isolates, multiplex PCR was performed in two groups: Group 1 (G1) consisting of isolates harboring vanA and vanC1 genes, and Group 2 (G2) comprising isolates positive for vanB and vanC2.3 genes (Table 1). The specificity of the primers was validated in NCBI database using Primer BLAST, which was performed using both primers and target template provided. The multiplex PCR reactions consisted of 10 pmol of each oligonucleotide primer (Table 1) and 12.5 µl master-mix red from Ampliqon, Denmark. The reaction conditions for van genes PCR were as follow: an initial denaturation at 94°C for 3 min, followed by 35 amplification cycles at 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min.
Table 1. Primers Sequences for PCR analysis and multiplex PCR for vanA, vanB, and vanC genes
| Gene | Oligonucleotide sequence (5’ to 3’) |
PCR product (bp) |
Reference | |
| ddl E. faecalis | F | ATCAAGTACAGTTAGTCTTTATTAG | 941 | (37) |
| R | ACGATTCAAAGCTAACTGAATCAGT | |||
| ddl E. faecium | F | TTGAGGCAGACCAGATTGACG | 657 | (37) |
| R | TATGACAGCGACTCCGATTCC | |||
| vanA | F | CATGACGTATCGGTAAAATC | 885 | (38) |
| R | ACCGGGCAGRGTATTGAC | |||
| vanB | F | CATGATGTGTCGGTAAAATC | 885 | (38) |
| R | ACCGGGCAGRGTATTGAC | |||
| vanC1 | F | GATGGCWGTATCCAAGGA | 467 | (38) |
| R | GTGATCGTGGCGCTG | |||
| vanC2.3 | F | GATGGCWGTATCCAAGGA | 429 | (38) |
| R | ATCGAAAAAGCCGTCTAC | |||
To investigate and compare the gene expression profiles associated with vancomycin resistance in E. faecium and E. faecalis, vancomycin-resistant and vancomycin-susceptible isolates were separately inoculated into 5 mL LB broth and incubated overnight at 37°C with agitation. A sub-inhibitory concentration of vancomycin was prepared using fresh LB broth for each bacterial isolate. The bacterial isolates were then inoculated in 25 mL fresh vancomycin-containing LB broth at 37°C with agitation until the mid-log phase of growth was reached (typically OD600 ≈ 0.6).
RNA extraction was performed on 10 E. faecalis and 10 E. faecium isolates using a commercial RNA extraction kit (High Pure RNA Isolation Kit, Roche, Germany). The vanA expression was conducted using SYBR Green quantitative real-time PCR (qRT-PCR) on a Rotor-Gene Q detection system (Qiagen, Germany) with an Ampliqon one-step RT-qPCR kit (Denmark). Amplification was carried out in final volume of 25 µL, along with 12.5 µL of the one-step RT-qPCR master mix. The reactions were performed under following conditions: 5 min at 95°C, followed by 35 cycles of 30 sec at 94°C, 32 sec at 52°C, and 10 sec at 72°C, concluding with a final extension of 3 min at 72°C.
The gene expression profiles between vancomycin-treated and vancomycin-untreated isolates of E. faecium and E. faecalis were analyzed and compared. The relative gene expression levels for the target genes were calculated by normalizing to the reference gene (16s rRNA).
The 16S rRNA primers used in this study were as follow: forward primer (F): “5`-CGCGGTGCATTAGCTAGTTG-3`”, and reverse primer (R): “5`-CCCTCTCAGGTGCGGCTAT-3`” (15).
Statistical Analysis
The relationship between the occurrence of vanA and vancomycin resistance of Enterococci isolates was analyzed using Fisher’s exact test. Statistical significance was determined at a significance level of less than 0.05 (P<0.05).
Ethical Considerations
We identified 82/120 (68.3%) E. faecalis, and 38/120 (31.6%) E. faecium. From 82 E. faecalis isolates, we obtained 56 (68.2%) from urine, 17 (20.7%) from tracheal samples, 7 (8.5%) from blood, and 2 (2.4%) from wound specimens. From 38 E. faecium isolates, we isolated 20 (52.2%) from urine, 6 (15.7%) from tracheal samples, 7 (18.4%) from blood, and 5 (13.16%) from wound specimens. Following this, PCR analysis tageting D-alanine-D-alanine ligases specific for E. faecalis (ddl E. faecalis) and E. faecium (ddl E. faecium) confirmed the phenotypic specification (Figure 1).
Antimicrobial Susceptibility Testing Results
The study revealed that resistance rates to ampicillin, penicillin, erythromycin, gentamicin, and ciprofloxacin among E. faecium isolates were as follows: ampicillin (89.5%), penicillin (84.2% ± 33%), erythromycin (73.7%), gentamicin (68.4%), and ciprofloxacin (63.1%). In contrast, the resistance rates among E. faecalis isolates were as follows: ampicillin (5.8%), penicillin (7.2%), erythromycin (41.7%), gentamicin (53.6%), and ciprofloxacin (39.5%). The results of antimicrobial susceptibility testing showed that 24 E. faecalis isolates and 13 E. faecium isolates were phenotypically resistant to vancomycin. It is important to note that vancomycin-resistant isolates were confirmed using E-test method. The resistance range for vancomycin-resistant E. faecium was typically expressed as 128-256 mg/L, and similarly, E. faecalis showed a resistance range of 64-256 mg/L.
Gene Responsible for Vancomycin Resistance
The study employed multiplex PCR method to amplify vanA, vanB, and vanC genes in both non-susceptible and susceptible isolates to vancomycin (Figure 1). The results showed that 13 (54.1%) resistant E. faecalis isolates and 9 (69.2%) resistant E. faecium isolates contained vanA gene. In contrast, all susceptible E. faecalis and E. faecium isolates lacked vanA, vanB, and vanC genes. A significant correlation was shown between vancomycin resistance and the presence of vanA gene (P<0.05) (Table 2).

Figure 1. Gel image of PCR products. Lane 1: Size marker (100 bp), Lane 2: ddl E. faecalis, Lane 3: ddl E. faecium, Lane 4: vanA, vanC1, Lane 5: vanB, vanC2,3, Lane 6: negative control.
Table 2. Correlation between genotype and disk diffusion result of antibiotics, along with their respective P values.
| Genotype/Antibiotic | P-value |
| vanA/Vancomycin | 0.008 |
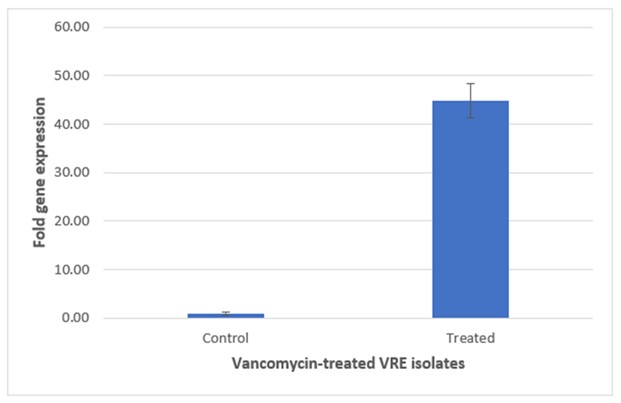
The respective expression of the vanA gene among vancomycin-treated and untreated VRE isolates was determined by using real-time RT-PCR and displayed as Relative Quantification (RQ) value. The mean±SEM of RQ values of vanA gene and fold-increase in the mean RQ values among VRE groups were shown in Figure 3. The RQ values for vanA in the treated VRE were significantly higher than those of the untreated vancomycin resistant Enterococci isolates. The respective RQ values of vanA expression in the treated VRE group were 8.6-fold of that in the untreated vancomycin resistant E. faecalis isolates.
Gene expression of 16S rRNA as housekeeping gene was evaluated in the presence and absence of the antibiotic vancomycin to establish its role in Enterococci resistance to vancomycin. Real-time PCR was performed using SYBR green. Ten vancomycin-resistant E. faecalis and E. faecium isolates with vanA gene were subjected to quantitative PCR analysis. Relative quantification was calculated as fold changes using delta-delta Ct method (Figure2).
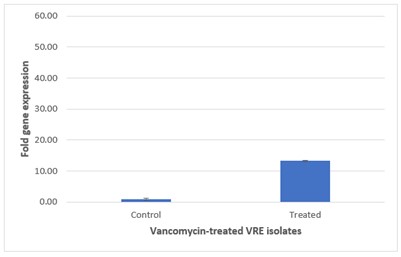
The relative quantification analysis revealed that the expression of vanA in vancomycin-treated E. faecium isolates was 2.6-fold higher than that observed in untreated resistant strains. This significant increase suggests that exposure to vancomycin may induce the expression of vanA gene, thereby potentially contributing to the resistance mechanisms in these isolates.
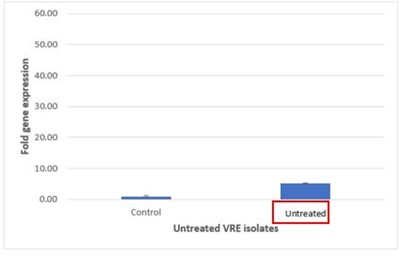
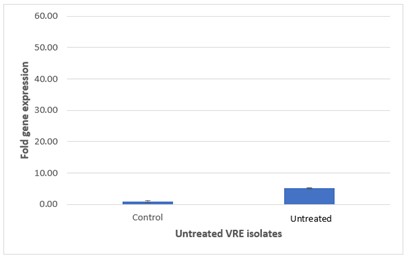
Figure 3 presents the comparative analysis of vanA gene expression between treated and untreated vancomycin-resistant E. faecium (VRE) groups. Figure 3 on top illustrates treated VRE group, where the elevated expression levels of the vanA gene are evident, while Figure 3 at Bottom depicts untreated VRE group, which shows lower expression levels. This graphical representation underscores differential gene expression resulting from antibiotic treatment.
Table 3 presents the results of gene expression study on VRE isolates. The study measured mean of 2DD, which is a quantitative measure of increase in bacterial mRNA during antibiotic treatment, for both untreated and treated VRE isolates. VanA gene expression levels in vancomycin-treated isolates were significantly higher compared to untreated isolates (P<0.001).
Table 3. Relative expression of vanA gene in vancomycin-resistant E. faecium.
| Antibiotic treatment | Mean of 2DD for control | Mean of 2DD for treated VRE isolates | Mean of 2DD for untreated VRE isolates | Relative quantification |
| E. faecalis | 0.93 | 44.84 | 5.11 | 8.6 |
| E. faecium | 0.93 | 13.28 | 5.11 | 2.6 |
In this study, we identified a total of 120 isolates, with 82 (68.3%) being E. faecalis and 38 (31.6%) being E. faecium. The majority of E. faecalis isolates were obtained from urine samples (56, 68.2%), followed by tracheal samples (17, 20.7%) and wound specimens (2, 2.4%), while the majority of E. faecium isolates were also isolated from urine samples (20, 52.2%), followed by tracheal samples (6, 15.7%) and blood samples (7, 18.4%). PCR analysis confirmed the phenotypic specification of these isolates by targeting D-alanine-D-alanine ligases specific for E. faecalis and E. faecium.
The isolation rates of E. faecalis and E. faecium in this study were similar to some other researches. For instance, a study by Boccella et al (17) identified 82.2% E. faecalis and 17.8% E. faecium isolates from 16 clinical samples. But another study by Sattari-Maraji et al (18) reported isolation rates of 68.8% E. faecalis and 31.2% E. faecium isolates. Notably, the prevalence of E. faecalis is consistently high across studies, with our results aligning closely with those of Sumangala et al (19), who reported an even higher percentage of E. faecalis at 88.1%. This suggests a general trend that E. faecalis is more frequently isolated from clinical contexts than E. faecium. Conversely, the study by Nasiri and Hanifian (20) reported lower percentages of E. faecalis (36.77%) and E. faecium (27.88%), indicating variability that could stem from differences in sample size, population characteristics, or geographical factors affecting microbial prevalence. Additionally, Georges et al (16) involved a smaller group of patients (n = 44) with the mean age of 37 years, which may influence the isolation rates due to demographic factors. The Ismail Hakki EKİN et al (21) highlighted the importance of specimen types, reporting isolates from both urine and stool, whereas the current study was performed on different specimen types. Such differences underscore the impact of local healthcare settings and practices on the observed prevalence of these organisms. Overall, while the predominance of E. faecalis appears to be a common theme, significant variations in the isolation rates and contexts in which data were collected, point to the necessity for the context-specific assessments in clinical microbiology.
The use of PCR analysis to confirm phenotypic specification is a common technique in microbiology research, as it provides a more accurate identification of bacterial species based on genetic markers rather than phenotypic characteristics alone, which can be influenced by environmental factors or antibiotic resistance mechanisms (22, 23). The use of D-alanine-D-alanine ligases as targets for PCR analysis is particularly useful for distinguishing between E. faecalis and E. faecium, as these enzymes are specific to each species and can provide a reliable identification method (24, 25).
The study revealed significant differences in antibiotic resistance between E. faecium and E. faecalis isolates. E. faecium showed higher resistance rates: ampicillin (89.5%), penicillin (84.2% ± 33%), erythromycin (73.7%), gentamicin (68.4%), and ciprofloxacin (63.1%). In contrast, E. faecalis exhibited much lower resistance rates: ampicillin (5.8%), penicillin (7.2%), erythromycin (41.7%), gentamicin (53.6%), and ciprofloxacin (39.5%). These findings indicate that E. faecium isolates are more resistant to these antibiotics than E. faecalis isolates.
Comparing the results of VRE in E. faecalis and E. faecium from various studies brings up a notable contrast in prevalence and resistance patterns. According to the review by Moghimbeigi et al (26) in 2018, E. faecalis was found to be more prevalent in clinical infections, representing 69% compared to 28% for E. faecium; however, the resistance to vancomycin was significantly higher among E. faecium strains (33%) than in E. faecalis strains (3%). This indicates that while E. faecalis is more commonly isolated in clinical settings, E. faecium poses a greater challenge concerning antibiotic resistance.
Similarly, Adeyemi et al (27) in 2021, reported that among 208 Enterococci strains, 85 (40.9%) were identified as VRE, with E. faecium accounting for 71.8% of these isolates. This trend reinforces the notion that E. faecium is increasingly associated with vancomycin resistance. Our findings also revealed phenotypic resistance to vancomycin in 24 E. faecalis isolates and 13 E. faecium isolates, underscoring the rising challenge of VRE in clinical contexts. There could be several reasons for the differences in vancomycin resistance rates and prevalence between our results and those of other studies. First, geographic variation is a significant factor; differences in local antibiotic usage, infection control practices, and healthcare settings can all influence the resistance patterns of Enterococci. For example, regions with higher antibiotic consumption may see increased levels of resistance. Second, sample size and composition can affect the generalizability of findings; our study sample size may differ from those of other studies, potentially leading to variations in detected resistance rates. Third, methodological differences in isolation and identification techniques, as well as in the criteria used for classifying resistance, can yield different outcomes. If different methods for phenotypic characterization of resistance are employed, this could account for discrepancies in results. Additionally, the presence of underlying health conditions in the patient populations being studied might differ, influencing the likelihood of encountering resistant strains. Lastly, temporal factors, like changes in resistance patterns over time due to evolving bacterial genetics or changes in the local epidemiology of infections, can also result in differing outcomes, highlighting the dynamic nature of antimicrobial resistance (18). The presence of the vanA gene in these isolates further highlights the importance of monitoring antimicrobial resistance patterns and implementing strategies to prevent the spread of resistant bacteria in clinical settings (17, 28, 29).
The study also found that among 24 resistant E. faecalis isolates and 13 resistant E. faecium isolates, phenotypic resistance to vancomycin was observed in both species; however, there was a statistical correlation between vancomycin resistance and the presence of vanA gene (P<0.05). Specifically, 75% of resistant E. faecalis isolates and 69.2% of resistant E. faecium isolates contained vanA gene; all susceptible E. faecalis and E. faecium isolates lacked vanA, vanB, and vanC genes, suggesting that these genes are associated with vancomycin resistance in both species studied herein.
Our findings regarding the correlation between vancomycin resistance and the presence of vanA gene in E. faecalis and E. faecium isolates were consistent with previous research. For instance, Resende et al (30) in their study found that all VRE isolates carry vanA gene (P<0.05).
Notable differences exist in prevalence of vanA gene among vancomycin-resistant Enterococci isolates across various studies that may originate from several factors. In the study by Mirzaei et al (31) in 2013, a relatively low prevalence of vancomycin resistance was observed, with only 13.6% of Enterococci isolates demonstrating resistance. Of these, E. faecalis and E. faecium accounted for 7.7% and 6.0%, respectively reflecting a moderate level of vanA gene detection. In contrast, Moosavian et al (32) in 2018, found a significantly higher vancomycin resistance rate of 43.4% among their Enterococcus isolates, with vanA gene detected in 91.5% of the resistant strains, which indicates a stronger association between vancomycin resistance and the presence of vanA gene in their isolates compared to our findings.
Our results showed that 54.1% of resistant E. faecalis and 69.2% of resistant E. faecium isolates contained vanA gene, suggesting a robust genetic basis for the resistance but still lower than what was reported by Moosavian et al (32).
Several factors may explain these discrepancies. Firstly, geographic variability and epidemiological factors play a crucial role; the studies may have been conducted in different regions with varying levels of antibiotic use, infection control practices, and patient demographics, leading to different resistance patterns. Secondly, study design and methodologies such as sample size, diagnostic criteria, and methods for identifying resistance genes can significantly impact the results. For instance, our study may have included a more selective patient population, or the methods for isolating and identifying VRE may differ, affecting detection rates of vanA gene. Furthermore, the potential influence of underlying health conditions or hospital-associated risk factors (e.g., use of central venous catheters, previous antibiotic exposure, etc.) could vary, leading to differences in infection types and severity, which might impact the prevalence of resistant strains. Lastly, temporal changes in resistance patterns due to evolving bacterial genetics or fluctuations in clonal spread could contribute to the observed differences, highlighting the need for continuous surveillance to understand the dynamics of antimicrobial resistance in enterococci (9).
The high prevalence of VanA gene among resistant isolates in both species highlights the importance of monitoring antimicrobial resistance patterns and implementing strategies to prevent the spread of resistant bacteria carrying these genes in clinical settings (30, 33, 34). The presence of these genes in both E. faecalis and E. faecium isolates also underscores the need for further research to understand the mechanisms by which these genes contribute to vancomycin resistance and to develop new antimicrobial therapies that can overcome this resistance (9).
The study also measured the expression of vanA gene among vancomycin-treated and untreated VRE isolates using real-time RT-PCR, and found that the mean RQ values for vanA expression in the treated group were significantly higher than those of untreated group, indicating higher levels of gene expression among treated isolates compared to untreated isolates. This finding suggests that the expression of VanA gene may contribute to vancomycin resistance in these bacterial species (15, 35).
The expression of vanA gene among vancomycin-treated VRE isolates was consistent with those reported in other studies. For instance, the expression of vanA gene was significantly correlated with vancomycin resistance in Aerococcus viridans (36).
The results of this study suggest that the expression of vanA gene, which is responsible for vancomycin resistance in Enterococci, is influenced by antibiotic therapy. Specifically, we observed a significant increase in the expression of vanA gene in VRE isolates treated with vancomycin compared to untreated VRE isolates. This finding is concerning as it suggests the use of vancomycin may actually promote the expansion of vancomycin-resistant Enterococci (VRE) through the up-regulation of vanA gene.
The study identified a high prevalence of vancomycin-resistant Enterococcus (VRE) isolates, with a majority showing resistance to vancomycin due to the presence of vanA gene. The resistance rates to various antibiotics were higher in E. faecium isolates compared to E. faecalis isolates. Moreover, the study demonstrated that the expression of vanA gene was significantly higher in VRE isolates treated with vancomycin. Overall, the research provides an in-depth exploration of the genetic changes and environmental factors influencing the emergence and spread of VRE, along with discussing potential treatment strategies to address this concerning pathogen and protect public health from antibiotic-resistant microorganisms.
We would like to thank Dr. A.D. for his advices and technical support.
Ethical Considerations
All procedures performed in this study was approved by the Ethics Committee of Islamic Azad University, Iran (Registration No.: IR.IAU.TNB.REC.1403.161).
Authors’ Contributions
G.Z.: study design, implementation, and funding. A.A.S, M.R.F. and, F.H.: scientific support. All authors contributed to the article and approved the submitted version.
The authors declared that there was no financial support for this work.
Conflicts of Interest
Received: 2024/10/10 | Accepted: 2025/01/9 | ePublished: 2025/01/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |