BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2408-en.html


 , Priti Agarwal1
, Priti Agarwal1 

 , Arundhati Biswas2
, Arundhati Biswas2 

 , Rajiv M. Gupta1
, Rajiv M. Gupta1 

 , Anil K. Pandey3
, Anil K. Pandey3 

 , Asim Das4
, Asim Das4 

 , Aparna Pandey5
, Aparna Pandey5 


2- Department of Paediatrics, ESIC Medical College and Hospital, Faridabad, India
3- Medical Superintendent, ESIC Medical College and Hospital, Faridabad, India
4- Dean, ESIC Medical College and Hospital, Faridabad, India
5- Department of Microbiology, World College of Medical Sciences & Hospital, Jhajjar, India ,
The onset of the COVID-19 pandemic has underscored a critical need for the comprehensive investigation into the surge of acute respiratory infections (ARIs) among paediatric populations. Such studies are indispensable for elucidating the multifaceted risk factors associated with these infections and devising targeted interventions and public health strategies tailored to safeguarding children's respiratory well-being in the aftermath of the pandemic. Since the first global COVID-19 pandemic triggered by SARS-CoV-2 around five years ago, different lineages have been evolving. Currently, the XBB and JN.1 Omicron lineages with enhanced transmissibility and immune escape are continuing to evolve. Information from surveillance contributed to the selection of the JN.1 and XBB.1.5 lineages as the target antigens during 2023–2024 and 2024–2025 (1).
In the late 2023, the JN.1 variety of SARS-CoV-2, a sub-clade of the BA.2.86 variant, was the predominant lineage in the United States, it quickly spread to 12 other countries, including Canada, France, and the United Kingdom (1).
The World Health Organization (WHO) recognized JN.1 different from BA.2.86 on December 20, 2023, and classified it as a Variant of Interest (VOI) due to its rapid dissemination. About 97.25% of the sequences reported to Global Initiative on Sharing All Influenza Data (GISAID) in India were of this type (1).
The JN.1 has a distinct L455S mutation in the receptor binding domain of the spike protein, which, increases transmissibility and immune evasion. Furthermore, monitoring variants like JN.1 are essential in limiting the spread of emerging variants (1).
Containment of the infections during the pandemic was possible due to imposition of the stringent pandemic protocols, including non-pharmacological interventions (NPIs) such as wearing mask and social distancing. These interventions disrupted customary social dynamics, potentially reshaping the landscape of respiratory infection vulnerability in children. While NPIs effectively curtailed the transmission of SARS-CoV-2 and concomitant respiratory illnesses in the early stages of the pandemic, the subsequent relaxation of these measures precipitated a resurgence of the acute respiratory infections among children (2).
A pivotal focus of interest lies in unravelling the intricate nexus between alterations in the social interaction patterns and the heightened susceptibility of children to the respiratory pathogens. Despite limited literature on the prevalence of respiratory viruses among hospitalized children with acute respiratory tract infections (ARIs) in India during the pandemic, a comprehensive understanding of the pandemic repercussions on the respiratory viruses’ epidemiology is imperative.
This study endeavours to bridge this knowledge gap by meticulously examining the impact of the COVID-19 pandemic on the epidemiology, frequency, and pattern of the respiratory viruses in hospitalized children with ARIs, shedding light on the crucial facets of paediatric respiratory health in the post-pandemic milieu.
Study Design, Setting, and Participants’ Selection
This cross-sectional study was conducted from February 2022 to December 2023 in the paediatric ward and neonatal intensive care unit (NICU) in a North Indian Hospital (ESIC Medical College and Hospital, Faridabad). The study enrolled symptomatic children younger than five years who were admitted to the NICU or paediatric units after exhibiting the respiratory symptoms fulfilling the case definitions. In order to maintain the focus on respiratory diseases other than COVID-19, only SARS-CoV-2-negative cases were included.
A total of 927 respiratory samples were included. To ensure the sample integrity, respiratory specimens were collected in accordance with accepted clinical practices for the specimen processing. The NP/OP specimens were collected within 48 hours of starting the antibiotic treatment using sterile flexible flocked nylon swabs. The samples were stored at 4°C for up to 24 hours, and then transferred to -70°C (3).
Case Definitions
The ARI was defined as any illness with fever, cough, nasal congestion, shortness of breath, or sore throat, with an acute onset (within seven days). Severe acute respiratory infection (SARI) was defined as patients requiring overnight hospitalization due to cough that started within the past seven days. For infants under two months, SARI included any physician-diagnosed acute lower respiratory infection necessitating the overnight hospitalization.
Data Collection
Each participant's environmental, clinical, and demographic information was documented. Age, gender, low birth weight, breastfeeding habits, indoor air pollution exposure, malnourishment, vaccination status, presence of smokers at home, and vitamin A supplementation were important factors. Clinical signs including fever, abnormal white blood cell (WBC) counts, antibiotic response, and detected respiratory infections were also recorded. Interviews with caregivers and medical records were used to collect data.
The study protocol was approved by the Institutional Human Ethics Committee (Approval number 134 X/11/13/2022-IEC/01) and demographic and clinical details of the patients were collected after obtaining written informed consent from the guardian of the participants. GraphPad Prism version 8.0 was applied to perform the statistical analysis. To assess the relationships between categorical data, like particular infections and clinical or demographic outcomes, the chi-square test was used. A P-value less than 0.05 was defined as statistically significant correlation between the variables.
Nucleic acid extraction and Real-Time PCR
Nucleic acids were extracted using the QIAamp Viral RNA Mini Kit (Hilden, Germany). The TRUPCR® Respiratory pathogen panel kit was used to detect 33 respiratory pathogens. The bacterial pathogens detected using the respiratory panel were Klebsiella pneumonia, Mycoplasma pneumoniae, Streptococcus pneumonia, Staphylococcus aureus, Acinetobacter baumanii, Bordetella species, Chlamydia pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, Pseudomonas aeruginosa, Legionella pneumophila, Haemophilus influenzae, and Moraxella catarrhalis. The viruses included parainfluenza 1-4, influenza A virus, (H3N2, Pandemic H1N1), influenzae B virus, human metapneumo virus (A+B), enterovirus, adenovirus, human respiratory syncytial virus, human rhinovirus, and human bocavirus.
Fluorophore-labelled probes specific for various targets were used. The amplification conditions consisted of cDNA synthesis followed by 40 cycles of PCR. Standard RT-qPCR reactions were performed with 20 μl of RT-qPCR mix containing master mix, primer/probe mix, enzyme, and extracted nucleic acid. The positive and negative controls were used to verify the reaction efficiency and to detect any contamination, respectively.
Patient Demographics
Children under the age of two years old comprised the majority of cases in the study. Among the mono-infections, 395 cases (234 males, 161 females) occurred in children younger than two years old, and 363 cases (261 males, 168 females) occurred in children aged two to five years. A total of 29 co-infections and 363 mono-infections were among children less than two years. Five children in the 2–5 years age group had co-infections, while 32 children had mono-infections (P=0.1981).
In regard to the gender distribution, males experienced a higher number of infections than females. Among mono-infections, 395 cases were reported in children aged less than 2 years, with 234 males and 161 females. In the 2-5 years age group, there were 363 cases, with 261 males and 168 females. For co-infections, 34 cases were recorded in children aged less than 2 years, with 27 males and 7 females. In the 2-5 years age group, there were 37 cases, with 27 males and 10 females.
Compared to females, males were substantially more likely to have both mono- and co-infections (P=0.0268). Mono-infections (13 cases) and co-infections (21 cases) were linked to low birth weight (P=0.16). Nine co-infections and seventy-one mono-infections were linked to lack of exclusive breastfeeding (P<0.0001). While exposure to indoor air pollution was associated with 33 co-infections and 91 mono-infections, crowded houses had 31 co-infections and 9 mono-infections with P=0.06 and P=0.003, respectively.
Malnutrition significantly correlated with respiratory infections, with 30 cases of co-infections and 122 cases of mono-infections (P<0.0001). Antibiotic response after 3-5 days showed a significant difference between co-infections (12 responded, 22 did not respond) and mono-infections (289 responded, 106 did not respond). Co-infections had a higher probability of not responding to antibiotics in the first three to five days (P<0.0001). Co-morbidities strongly correlated with increased risks of co-infections (P<0.0001). HIV infection was significantly associated with greater co-infection rates (P=0.0002).
Pneumonia was documented in 26/395 (6.58%) mono-infection and 31/34 (91.18%) of co-infection cases. Pneumonia had a higher frequency in co-infections than in mono-infections (P<0.0001). Rhinitis was documented in 354/395 (89.62%) and 12/34 (35.29%) of mono-infection and co-infection cases, respectively. Pharyngitis was documented in 298/395 (75.44%) mono-infection and 25/34 (73.53%) co-infection cases. Rhinitis and pharyngitis had a strong correlation (P<0.0001) with mono-infections.
Bronchiolitis was documented in 334/395 (84.56%) mono-infection cases and 28/34 (82.35%) co-infections. Sore throat was documented in 363/395 (91.90%) mono-infection cases and 19/34 (55.88%) co-infections (P=0.8048). Acute otitis media was documented in 148/395 (37.47%) mono-infection cases and 31/34 (91.18%) co-infections (P<0.0001). The prevalence of mono or co-infections did not significantly correlate with low birth weight, crowded house, fever, abnormal WBC count, immunization, or diarrhoea. Details are illustrated in Table 1.
Table 1. Demographic data, diagnosis, clinical characteristics, and demographics of enrolled patients stratified under mono-infections and co-infections
| Variable | Group | mono-Infections (n=395) | co-Infections (n=34) | P-Value | Odds Ratio (OR) |
| Age Group | < 2 years | 363 | 29 | 0.1981 | - |
| 2-5 years | 32 | 5 | |||
| Sex | Male | 234 | 27 | 0.0268 | 1.52 |
| Female | 161 | 7 | |||
| Low Birth Weight | Yes | 289 | 21 | 0.1648 | 1.43 |
| No | 106 | 13 | |||
| Lack of Exclusive Breastfeeding | Complete | 324 | 9 | <0.0001 | 12.8 |
| Never | 71 | 25 | |||
| Crowding in Household | Yes | 386 | 31 | 0.0611 | 2.4 |
| No | 9 | 3 | |||
| Exposure to Indoor Air Pollution | Yes | 304 | 33 | 0.0038 | 12.6 |
| No | 91 | 1 | |||
| Malnutrition | Yes | 122 | 30 | <0.0001 | 16.8 |
| No | 273 | 4 | |||
| Immunization | Yes | 126 | 11 | 1.0000 | 0.9 |
| No | 269 | 23 | |||
| Smokers in Household | Yes | 356 | 29 | 0.3745 | 0.79 |
| No | 39 | 5 | |||
| Vitamin A Supplement | Received | 112 | 14 | 0.1203 | 1.73 |
| Not Received | 283 | 20 | |||
| Fever | Yes | 367 | 29 | 0.1667 | 1.38 |
| No | 28 | 5 | |||
| Abnormal WBC Count | Abnormal | 307 | 30 | 0.1929 | 2.15 |
| Normal | 88 | 4 | |||
| Antibiotic Response (3-5 Days) | Response Seen | 289 | 12 | <0.0001 | 6.1 |
| No Response | 106 | 22 | |||
| Presence of Co-Morbidities | Yes | 109 | 28 | <0.0001 | 12.2 |
| No | 286 | 6 | |||
| HIV Co-Infection | Yes | 4 | 5 | 0.0002 | 16.8 |
| No | 391 | 29 | |||
| Diarrhoea | Yes | 108 | 12 | 0.3241 | 1.2 |
| No | 287 | 22 | |||
| Pneumonia | Yes | 26 | 31 | <0.0001 | 50.2 |
| No | 369 | 3 | |||
| Rhinitis | Yes | 354 | 12 | <0.0001 | 4.5 |
| No | 41 | 22 | |||
| Pharyngitis | Yes | 298 | 25 | 0.8363 | 0.9 |
| No | 97 | 9 | |||
| Bronchiolitis | Yes | 334 | 28 | 0.8048 | 0.88 |
| No | 61 | 6 | |||
| Sore Throat | Yes | 363 | 19 | <0.0001 | 3.6 |
| No | 32 | 15 | |||
| Acute Otitis Media | Yes | 148 | 31 | <0.0001 | 17.3 |
| No | 247 | 3 | |||
P<0.05 are considered significant by Chi-square test
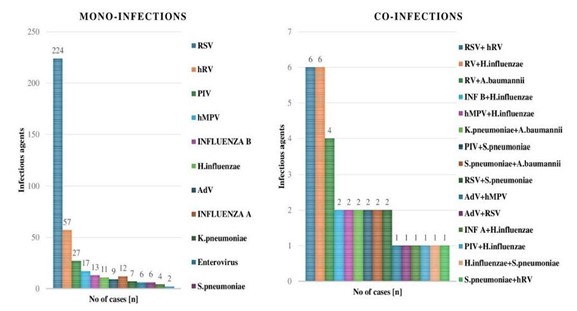
Figure 1. Distribution of mono-infections and co-infections among the ARI cases.
Seasonal Distribution
These findings reveal the seasonal fluctuations in respiratory viruses’ prevalence observed during the two-year study period. In our investigation, influenza A H1N1 demonstrated sporadic activity, particularly notable in August (50% positivity) and September (38% positivity). In contrast, influenza A H3N2 peaked in March (75% positivity), with smaller peaks in August (25% positivity) and December (12% positivity). Influenza B exhibited sporadic activity, with a significant peak in October (62% positivity). RSV displayed a seasonal pattern, with peaks during colder months such as March (25% positivity), April (15% positivity), and December (12% positivity). hMPV cases peaked in December (59% positivity), with smaller peaks in April (30% positivity). PIV cases were the highest in December (40% positivity), followed by November (11% positivity). RV cases peaked in November (48% positivity), with smaller peaks in December (23% positivity) and August (4% positivity). AdV cases peaked in December (56% positivity), with significant activity also observed in November (33% positivity). While hMPV cases remained relatively stable throughout the observation period, RSV cases spiked in 2023 compared to 2022. The number of PIV infections fluctuated over time, with 2023 showing an increase in instances relative to 2022. hRV instances were consistently reported throughout the observation period. Although Adv events were generally low, there was some activity in both years (Figure 2).
The data also indicates a decline in COVID-19 prevalence from 2022 to 2023, despite the virus remaining a significant concern.
This suggests the effectiveness of immunization efforts and public health initiatives, as well as the potential build-up of immunity among the population. However, other respiratory diseases such as RSV, hMPV, PIV, and INF H3N2 exhibited variable activity levels that varied somewhat from year to year. The presence of these respiratory illnesses underscores the on-going challenges in managing infectious diseases beyond COVID-19. Continuous surveillance and public health interventions are crucial for monitoring and controlling the spread of these respiratory illnesses, especially considering viral evolution and potential seasonal trends (Figures 3 and 4).
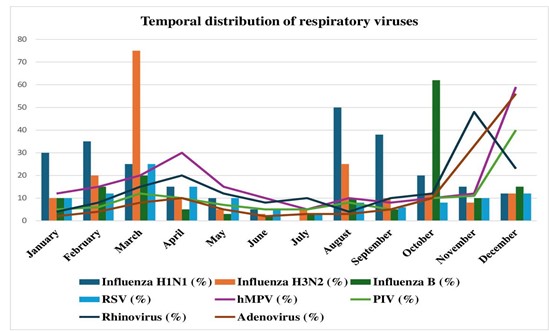
Figure 2. Temporal distribution of respiratory viruses during the study.

Figure 3. Month-wise positivity trend of respiratory viruses during pandemic and post- pandemic with respect to SARS-CoV-2 virus (4).
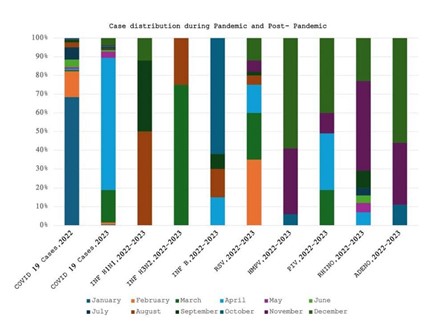
Figure 4. Month-wise positivity trend of respiratory viruses before and after SARS-CoV2 pandemic (5, 6).
Respiratory viruses’ positivity trend preceding during and following SARS-CoV-2 pandemic
A comprehensive investigation of the respiratory viruses’ positive trends before, during, and after the SARS-CoV-2 epidemic revealed notable variations in the virus circulation. Different levels of influenza A H3N2, influenza A H1N1, influenza B, RSV, hMPV, hRV, and PIV infections were reported in Kang et al (4), pre-pandemic investigation.
It is noteworthy to mention that, with 85 cases reported, RSV was the most common virus during this time. But as Kang et al., study showed, there was a significant drop in respiratory viruses’ positivity during the pandemic period, with low detections of most viruses aside from occasional occurrences of influenza A H1N1, hRV, and PIV (7, 8).
Fascinatingly, our analysis from 2022 during the pandemic era revealed a significant revival in respiratory viruses’ positivity, specifically for RSV and influenza A H1N1, suggesting that respiratory viruses circulation may resume even with on-going pandemic preventive measures. In contrast to the pandemic period, our study findings in 2023 showed a significant rise in respiratory viruses’ positivity for all viruses, indicating a return to the pre-pandemic levels of respiratory viruses’ circulation (Figure 3). With a notable increase in cases, RSV resurfaced as the most common virus, underscoring the dynamic nature of the respiratory viruses’ epidemiology across time.
These results underscore the importance of continually keeping a watch out for the respiratory viruses’ outbreaks and being prepared to combat them both during and after pandemic periods in order to efficiently mitigate their adverse impacts on public health.
The study highlights key findings regarding acute respiratory infections (ARI) in paediatric populations. Out of total cases, 386 ARI cases originated from the paediatric wards, while 43 cases were from intensive care units (ICUs), predominantly affecting infants, particularly males. This observation aligns with previous research by McClelland et al (7), Juliana et al (8), and Zhao et al (9) suggesting gender-specific immune response differences may contribute to this trend. Real-time PCR analysis revealed 34 cases with co-infection and 395 cases with mono-infection, emphasizing the complexity of ARI cases and the potential for multiple pathogen involvement simultaneously, consistent with existing literature (10-12).
The seasonality of respiratory viruses, such as influenza, human coronaviruses, and RSV demonstrates winter peak episodes, while adenovirus, human bocavirus, rhinovirus, and hMPV are present year-round. According to studies by Peci et al (13), Midgley et al (14), Killerby et al (15), Monto et al (16), Landes et al (17), Morikawa et al (18), Bastien et al (19), Haynes et al (20), Abedi et al (21), Lee et al (22), and Monto (23), the enteroviruses, known as "summer viruses," exhibit increased counts during warmer months, while rhinovirus severity peaks in winter. Although the findings of the current study on RSV align with previous studies, the higher incidence of human metapneumovirus could be due to variations in the respiratory viruses’ epidemiology, variations in the study populations, or advancements in the diagnostic techniques as suggested by Falsey et al (24), Atmar et al (25), Li et al (26), and Suminda et al (27).
The COVID-19 pandemic altered the seasonality of influenza and RSV infections globally, with off-season spikes following declines during COVID-19 peaks (28-30).
Several theories attempt to explain the decrease in influenza and RSV cases during SARS-CoV-2 peaks, including viral antagonism and NPIs. Post-pandemic influenza subtypes have shifted with changes in circulation patterns observed (31).
Studies in India during the pandemic and post-pandemic era highlight shifts in the respiratory pathogens circulation, with varying prevalence and seasonality observed (4-6). Our study reflects these trends, with clear seasonal patterns in respiratory viruses’ prevalence, particularly during colder months. RSV, PIV, and adenovirus peak towards the end of the year, while influenza A H3N2 peaks in November and influenza B in October. Rhinovirus and hMPV exhibit intermittent activity throughout the year, with sporadic peaks. Overall, our findings contribute to understanding the dynamic nature of respiratory viruses’ circulation and the impact of the COVID-19 pandemic on seasonal patterns and prevalence.
The study limitations include its restricted generalizability to other age groups or geographical areas due to the small sample size. Recall bias and the use of self-reported information and medical records may result in potential errors. Furthermore, knowledge of infection dynamics and the discovery of possible biomarkers are impeded by the lack of immunological analysis and the limited investigation of long-term health repercussions, which ultimately impacts the formulation of the successful treatment plans.
The study found a strong association between the acute malnutrition and early ARIs, supporting previous research that identifies malnutrition as a key contributor to the childhood ARIs. This link may stem from malnutrition role in compromising immune function, increasing vulnerability to the infections (32, 33).
Additionally, the detrimental effects of inadequate breastfeeding on child health highlight the need for the targeted health promotion campaigns. Such initiatives should focus on providing support for the breastfeeding through community health workers, lactation consultants, and support groups.
The study underscores significant associations between various demographic factors and ARI prevalence, including age, gender, low birth weight, breastfeeding practices, household conditions, exposure to indoor air pollution, and malnutrition. These findings highlight the multifactorial nature of the ARI and emphasize the importance of addressing socio-environmental determinants in disease prevention and management. By examining the viral evolution dynamics and its correlation with respiratory infections epidemiology in children, the study aimed to anticipate and address the emerging threats to paediatric respiratory health in the post-pandemic period. Given the on-going repercussions of the COVID-19 pandemic, it is crucial to comprehensively examine the risk factors for the ARIs in children. This research contributes to understanding the multifaceted aspects of the social interaction, immune system alterations, and viral evolution, aiming to inform targeted interventions and public health strategies to safeguard children's respiratory health in the post-pandemic era. Understanding the impact of post-pandemic respiratory infections on children requires a thorough exploration of the various involved risk factors. As we delve deeper into these dynamics, it becomes increasingly clear that a holistic approach is essential for developing effective interventions and public health strategies.
The authors would like to acknowledge the study participants for their cooperation.
Ethical Considerations
The study protocol was approved by the Institutional Human Ethics Committee (Approval number 134 X/11/13/2022-IEC/01). Demographic and clinical details of the patients were collected from the guardians of the participants after obtaining written informed consent.
Authors’ Contributions
Aparna Pandey conceptualized, supervised, and administered the study. Arundhati Biswas and Priti Agarwal conducted the formal analysis. Pooja Pandey, Aparna Pandey, Anil K Pandey, Rajiv M Gupta and Asim Das conducted the investigation. Priti Agarwal, Arundhati Biswas and Pooja Pandey wrote the main manuscript text and edited. All authors reviewed and approved the manuscript.
There is no grant support or financial relationship associated with this study
Conflicts of Interest
Received: 2024/07/24 | Accepted: 2024/11/11 | ePublished: 2024/11/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





