BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2399-en.html
2- Department of Biology, College of Science, University of Diyala, Diyala, Iraq
3- Department of Internal Medicine, College of Medicine, University of Diyala, Diyala, Iraq
The most important causes of asthma, blood poisoning, meningitis, and wound and urinary tract infections (UTIs) are A. baumannii bacteria (1). These opportunistic bacteria evolved to multidrug-resistant (MDR) and extensively drug-resistant (XDR) (2). The emergence of resistance to different types of antibiotics has become a common cause of problems in choosing the appropriate treatment (3). These bacteria are resistant to numerous antibiotics such as amoxicillin, cephalosporin, cefotaxime, chloramphenicol, gentamicin, tobramycin, quinolones, and macrolides (4, 5). Biofilm formation is the most important virulence factor that contributes to the adaptation of bacteria to survive and spread infections (6). Microorganisms that form biofilm can cause persistent diseases and are antibiotic-resistant (7). The poly-(1,6)-N-acytelglucose amine is an additional polysaccharide hypothesized to function as an intracellular adhesion factor for the biofilm formation of bacteria (8, 9). Resistance to antibiotics, especially aminoglycosides, has increased due to the increased use of antibiotics (10).
There are two main causes of aminoglycoside resistance: the entry of aminoglycoside antibiotics and methylation enzymes (11). They are controlled by 12 different genes (12). The A. baumannii may move by excreting exopolysaccharide, a film consisting of sugar chains with high molecular weight (13). The oxidase test was used by clinical microbiologists to differentiate Acinetobacter spp. from other Moraxellaceae. The Acinetobacter spp. are the only Moraxellaceae members with lack of cytochrome c oxidases (14). The ACB complex includes (A. baumannii, A. calcoaceticus, and Acinetobacter genomic species 13TU). Members of the ACB complex are therapeutically relevant but difficult to identify (15). Most nosocomial infections are caused by A. baumannii, an ESKAPE pathogen (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) (16). In European intensive care centers, infections caused by Acinetobacter spp. accounted for 21.8% of pneumonia cases, 17.1% of bloodstream infections, and 11.9% of UTIs (17). The pathogenic characteristics of A. baumannii include pathogenicity islands that are groups of two or more genes that enhance the virulence of a disease (18, 19). They can express toxins, coagulate blood, or cause resistance to antibiotics. AbaR-type resistance islands, which lead to drug resistance, are common in A. baumannii. Each one is 16.3 Kb and promotes horizontal gene transfer. Transposons allow the genetic material to be moved across the genomic locations (20).
Dual efflux pumps in A. baumannii reduce its antibiotic susceptibility. The first pump, AdeB, is aminoglycoside resistant (21), and the second one, known as AdeDE, is responsible for the efflux of a wide variety of substrates. These substrates include tetracycline, chloramphenicol, and other carbapenems (22). This study aimed to extract A. baumannii from UTIs and detect biofilm-forming bacteria from the clinical specimens.
The bacteria were isolated from collected samples during the period of August 2022 to February 2023. The samples (130 urine samples) were obtained from Al Batool Hospital and Al-Jumhuri Teaching Hospital. These samples were incubated directly on blood agar and MacConkey agar at 37°C for 24 hr in order to initial diagnosis and knowledge of bacterial growth conditions. Then, suspected bacteria were cultured on selective Acinetobacter medium (LAM) (7-8) to confirm the diagnosis. Subsequently, all samples were confirmed by VITEK2 and molecular examination.
Identification of the Isolated Bacteria
Cultural Characteristics and Microscopic Evaluation
The staining and cell arrangement were observed under the light microscope. Morphological examination was combined with biochemical testing to specifically identify the isolates. The oxidase, urease, and motility tests were conducted, as well as oxidation–fermentation (O-F) tests, catalase tests, and IMViC tests (Indole test, Methyl Red test, Voges-Proskauer test, and Citrate test), triple sugar iron Agar, and carbohydrate fermentation tests according to the instructions (9, 10).
Identification of A. baumannii by PCR Amplification
According to the previous studies (11, 12) the salting out approach was used to extract DNA from the isolates. DNA was taken from a patient's blood and used as a template for polymerase chain reaction (PCR) amplification. The mixtures (25 μl) consisted of GoTaq® Green master mix 2X (Promega, USA) and 10 μM of each forward (AGAGTTTGATCCTGGCTCAG) and reverse (TACCAGGGTA TCTAATCCTGTT) primers (Kapa, USA) (13), DNA template (100 μg) and PCR free ion water (Promega). The 16S ribosomal RNA (rRNA) was amplified using a thermal cycler (Gene Amp 97000, Applied Biosystem-Singapore).
To replicate 16S rRNA the cycling program below was followed: initial 95⁰C denaturation for 3 min, 30 cycles of 95⁰C for 1 min, 55⁰C annealing for 1 min, 72⁰C extension for 1 min, and a final 72⁰C extension of 5 min. To identify the PCR amplicons, the PCR products were exposed to agarose gel electrophoresis, and visualization was performed on the gels containing ethidium bromide.
Biofilm Formation
Tryptic soy broth (TSB) was inoculated into the sterile 96-well flat-bottomed plates loaded with bacterial culture (Hi Media India). Negative controls utilized only medium for 24 hr at 37°C. Each well was then drained, rinsed with sterile saline, and dried. The plates were then incubated for 5 min with 2% crystal violet (Hi Media India). Any residual stain on the plate was washed away with dH2O. After that, the plates were air-dried, and the dyes conjugated to the adhering cells were solubilized with 33% glacial acetic acid (v/v). An ELISA reader was used to measure the optical density (OD) at 650 nm (10, 11).
Detection of Extended-Spectrum β-lactamases Production
The double disk synergy test (DDST), was performed in accordance with the procedures previously described (23) to establish the level of extended-spectrum β-lactamases (ESBL) production.
Detection of Metallo β-lactamases Production
After placing two disks of 10 μg imipenem (IMP) on the surface culture already contained the isolates, ethylenediaminetetraacetic acid (EDTA) (0.5 M) and dehydrated disodium salt (750 μg) were added to one IMP disk. Following 16-18 hr incubation, a comparison was made between the inhibitory zones surrounding IMP and EDTA disks. A favorable result would be the rise of at least 7 mm in the zone size surrounding the IMP-EDTA disk (14).
Antimicrobial Susceptibility Tests and Examination of MDR and XDR Isolates
Disk diffusion method was used to determine the MDR and XDR levels of the local isolates of A. baumannii using Mueller Hinton agar and antibiotic disks ampicillin-sulbactam (20 μg), meropenem (10 μg), clarithromycin (15 μg), cefetriaxone (30 μg), azithromycin (15 μg), erythromycin (15 μg), nalidixic acid (30 μg), Trimethoprime (5 μg), levofloxacine (5 μg), ciprofloxacine (5 μg), amikacin (30 μg), gentamicin (10 μg), Piperacillin (30 μg), imipenem (30 μg), tetracycline (30 μg), chloramphenicol (10 μg), cefoxitin (30 μg) and ceflazidime (30 μg) as mentioned in CLSI 2019 guidelines (18, 19).
Statistical Analysis
The data from the shaking and static microtiter plates and tube techniques were compared using SPSS 20 and one-way ANOVA statistical technique. A P-value less than 0.05 in the repeated tests was considered significant.
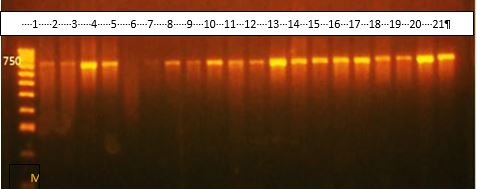
Only 20 isolates of A. baumannii from 130 patients with clinical UTI symptoms were identified and diagnosed by morphological, biochemical, and VITEK2 system criteria. All isolates were catalase-positive and oxidase-, urease-, and sugar fermentation-negative, as shown in Table 1. In addition, PCR with A. bumannii 16S rRNA gene primers confirmed all the isolates, as shown in Figure 1.
Table 1. The biochemical tests for A. baumannii identification
| Biochemical test | Result |
| Oxidase test | - |
| Galactose | + |
| Catalase test | + |
| Triple sugar iron agar test (TSI) | K/K, H2S - |
| Oxidation Fermentation Test | + |
| Motility test | - |
| Glucose | + |
| Urease test | - |
| Fructose | - |
| IMVIC | + --- |
| Sugar fermentation test | - |
| Sorbitol | - |
| Sucrose | - |
| Maltose | - |
| Lactose | - |
Figure 1. 16S rRNA gene PCR amplification results (product size 750 bp). Lane 1: DNA marker (1500 bp); Lanes 2-21: bacterial isolates with positive results except for Lane 6.
Antibiotic Susceptibility
Table 2 shows antibiotic susceptibility of A. baumannii isolates. Only one (5%) isolate was responsive to ampicillin and trimethoprim-sulfamethoxazole versus 19 (95%). Just two (10%) isolates were sensitive to cefepime from cephalosporines and nitrofurantoin, while 18 (90%) were resistant to the same antibiotic. Three isolates (15%) were responsive to naldixic acid versus 17 (85%) were resistant. Only four (20%) isolates were sensitive to ceflazidime, while 16 (80%) were resistant to the same antibiotic. In addition, there were 6 isolates (30%) sensitive to cefotaxime, cefoxitin, gentamicin, azithromycin, and chloramphenicol, while 14 isolates (70%) were resistant to the same antibiotics. Piperacillin and tetracycline resistance were 13 (65%). In addition, the current study recorded resistance rates of 60%, 50%, and 30% to amoxicillin-clavulanate, imipenem, and meropenem, respectively. In this study, 4 isolates (20%) were resistant to ciprofloxacin and levofloxacin.
Table 2. Antibiotic susceptibility of A. baumannii isolates.
| Antibiotics | Sensitive | Resistant | ||
| No | % | No | % | |
| Ampicillin | 1 | 5 | 19 | 95 |
| Trimethoprim-sulfamethoxazole | ||||
| Cefepime | 2 | 10 | 18 | 90 |
| Nitrofurantoin | ||||
| Naldixic acid | 3 | 15 | 17 | 85 |
| Ceflazidime | 4 | 20 | 16 | 80 |
| Cephotoxime | 6 | 30 | 14 | 70 |
| Cefoxitin | ||||
| Gentamicin | ||||
| Azithromycin | ||||
| Chloramphenicol | ||||
| Pipracillin | 7 | 35 | 13 | 65 |
| Tetracycline | ||||
| Amoxicillin – Clavulanate | 8 | 40 | 12 | 60 |
| Imipenem | 10 | 50 | 10 | 50 |
| Meropenem | 14 | 70 | 6 | 30 |
| Ciprofloxacin | 16 | 80 | 4 | 20 |
| Levofloxacin | ||||
Multidrug-Resistance of A. baumannii
The current study found that 14 out of 20 (70.0%) and 6 out of 20 (30.0%) A. baumannii isolates were MDR XDR, respectively.
Table 3. MDR and XDR isolates.
| Categories | % |
| Multidrug-resistant (MDR) | 70.0 |
| Extensively drug-resistant (XDR) | 30.0 |
Association of Biofilm Formation With Variables
The results demonstrated that 11 (55%) of the 20 isolates were strong and moderate biofilm formers, while 3 (15%) were non-producers (Table 4).
Table 4. The highest rates of biofilm formation in general
| Biofilm formation of total No. isolates (20) | Biofilm formation | |
| No. | % | |
| Strong and Moderate | 11 | 55 |
| Non-biofilm | 3 | 15 |
Association of Biofilm Formation With Antibiotic Susceptibility
Table 5 reveals that all strong biofilm former isolates resisted all drugs except meropenem, ciprofloxacin, and levofloxacin. No statistically significant differences were observed (P˃0.05).
Table 5. Association of biofilm formation with antibiotic susceptibility.
| Antibiotics | Biofilm formation | ||||||
| Strong | Moderate | Non-biofilm | |||||
| No. | % | No | % | No | % | ||
| Ampicillin | Sensitive | 1 | 5 | - | - | - | - |
| Resistant | 6 | 30 | 11 | 55 | 2 | 10 | |
| Pipracillin | Sensitive | 1 | 5 | 4 | 20 | 2 | 10 |
| Resistant | 8 | 40 | 5 | 25 | - | - | |
| Amoxacillin-Clavunate | Sensitive | 3 | 15 | 3 | 15 | 2 | 10 |
| Resistant | 7 | 35 | 5 | 25 | - | - | |
| Cefepime | Sensitive | 1 | 5 | 1 | 5 | - | - |
| Resistant | 10 | 50 | 6 | 30 | 2 | 10 | |
| Cephotoxime | Sensitive | 3 | 15 | 3 | 15 | - | - |
| Resistant | 6 | 30 | 6 | 30 | 2 | 10 | |
| Cefoxitin | Sensitive | 3 | 15 | 2 | 10 | 1 | 5 |
| Resistant | 6 | 30 | 7 | 35 | 1 | 5 | |
| Ceflazidime | Sensitive | - | - | 2 | 10 | 2 | 10 |
| Resistant | 11 | 55 | 5 | 25 | - | - | |
| Imipenem | Sensitive | 4 | 20 | 4 | 20 | 2 | 10 |
| Resistant | 7 | 35 | 3 | 15 | - | - | |
| Meropenem | Sensitive | 5 | 25 | 7 | 35 | 2 | 10 |
| Resistant | 3 | 15 | 2 | 10 | 1 | 5 | |
| Gentamicin | Sensitive | 2 | 10 | 4 | 20 | - | - |
| Resistant | 8 | 40 | 5 | 25 | 1 | 5 | |
| Azithromycin | Sensitive | 2 | 10 | 3 | 15 | 1 | 5 |
| Resistant | 9 | 45 | 5 | 25 | - | - | |
| Tetracycline | Sensitive | 3 | 15 | 3 | 15 | 1 | 5 |
| Resistant | 7 | 35 | 6 | 30 | - | - | |
| Ciprofloxacin | Sensitive | 6 | 30 | 9 | 45 | 1 | 5 |
| Resistant | 3 | 15 | 1 | 5 | - | - | |
| Levofloxacin | Sensitive | 7 | 35 | 7 | 35 | 2 | 10 |
| Resistant | 2 | 10 | 2 | 10 | - | - | |
| Nalidixic acid | Sensitive | 1 | 5 | 2 | 10 | - | - |
| Resistant | 9 | 45 | 7 | 35 | 1 | 5 | |
| Trimethoprim-sulfamethoxazole | Sensitive | - | - | 1 | 5 | - | - |
| Resistant | 7 | 35 | 9 | 45 | 3 | 15 | |
| Chloramphenicol | Sensitive | 4 | 20 | 2 | 10 | - | - |
| Resistant | 5 | 25 | 8 | 40 | 1 | 5 | |
| Nitrofurantoin | Sensitive | - | - | 2 | 10 | - | - |
| Resistant | 10 | 50 | 7 | 35 | 1 | 5 | |
The origin of bacterial resistance is due to the ability of the bacteria to produce beta-lactamase enzymes or due to a change in the target site and a decrease in permeability of the outer wall (24). The efflux systems may also expel anticellular agents to the outside (25). Microorganisms resistant to at least one antibacterial treatment of three or more are also referred to as multidrug-resistant (MDR). However, when a bacterial species is resistant to all but fewer than two antimicrobial antibiotics, it is called extensively drug-resistant (XDR) (26).
The current study results indicate that the majority of A. baumannii isolates were MDR. These strains are rapidly extended between hospitalized patients. These findings are matters of concern for the healthcare staff and society, as they make treating these bacteria complicated. It is consistent with a 2019 CDC report on antibiotic resistance in which the study classified carbapenem-resistant Enterobacteriaceae and the resulting risk as an urgent issue and that addressing these threats requires a collaborative global public health approach (27).
Resistance genes are passed by mobile genetic elements such as insertion sequences (IS), plasmids, transposons, and gene cassettes in integrons (28). As well as, which conjugative transposons can transport wide range of resistance genes which allowing spreading multidrug resistance between different types of bacteria (22). In Acinetobacter, resistance to cephalosporins and carbapenems is strongly correlated with IS, many of ISAba elements have ability to encode potent externally facing promoters which are required for expression of β-lactamases to confer clinical resistance (29).
The A. baumannii antibiotic resistance can be caused by inappropriate exposure to antibiotics, chronic widespread use of antibiotics, exposure to resistance genes, or lack of clinical hygiene. Also, due to the extreme consumption of quinolones and aminopyrimidine, the emergence of resistant strains has caused a lot of concern in treating transferable resistance.
The receptor of A. baumannii isolates may be both XDR and pan drug-resistant (PDR). The A. baumannii is resistant to carbapenems and other antibiotics (30).
Biofilms that are common in nature for bacterial survival are responsible for their antibiotic resistance. Biofilms contribute to the pathogenesis of bacterial diseases, especially in chronic infections (31, 32). Bacteria can cause tissue damage and severe infections by hiding from the immune system. In biofilms, bacteria adapt to hypoxia and nutritional deficiencies by altering their metabolism, protein production, and gene expression, which may reduce their metabolic rate and cell division rate (33).
Modifications such as inactivating the antimicrobial targets or reducing the requirements for cellular function can render the bacteria resistant to antimicrobial treatment (34). These are plausible explanations for the increased antibiotic resistance of most A. baumannii isolates in the current study, even if the increase is not statistically significant. Biofilms of microorganisms are embedded in a self-produced extracellular matrix that clings to a biotic or abiotic surface. The pathogenic biofilms can exist in high concentrations of antibiotics (35). Biofilms acquire antibiotic resistance through various processes, some of which can increase the number of MDR bacteria (36, 37). Biofilms are a reservoir of antibiotic resistance genes.
This study found that A. baumannii isolates more resistant to antibiotics were more strong biofilm formers, and the origin of resistance is due to the capacity of bacteria to produce broad-spectrum beta-lactamase enzymes EsβLs. We also noticed that the best antibiotics with the ability to eliminate clinical strains of A. baumannii are ciprofloxacin and levofloxacin. It is recommended that physicians and health workers in Diyala use them for the treatment of the patients infected with MDR A. baumannii.
We appreciate our colleagues in the College of Medicine, University of Diyala for their lab facilities and the staff at Al Batool Hospital and Al-JUmhuri Teaching Hospital for their hard work, support, and help in collecting information.
Ethical Approvel
The Health Department in Diyala Governorate approved the research protocol, which is affiliated with the Ministry of Health in Iraq. Special consent forms were designed to obtain the patient's consent before taking the sample. The signed informed consent assured confidentiality.
Authors’ Contributions
Conceptualization: Hanan Raheem Hassooni; Methodology: Raghad Ibrahim Ahmed; Statistical analysis: Hanan Raheem Hassooni; Preparation: Zainab M. Alzubaidy; Editing: Raghad Ibrahim Ahmed and Adil Hassan Alhusseiny
The author(s) received no financial support for the research or publication of this article.
Conflicts of Interest
No conflicts of interest were declared by the authors.
Received: 2024/04/24 | Accepted: 2024/08/8 | ePublished: 2024/08/18
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |