BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2393-en.html
2- Department of Microbiology, Sree Balaji Medical College and Hospital, Bharath Institute of Higher Education and Research, Chennai, India
The emergence of carbapenemase enzymes within the Enterobacteriaceae family presents a significant and increased challenge to the global public health, which are known for their high incidence, multidrug resistance, a wide range of clinical diseases, as well as rapid transmission of plasmid-mediated resistance genes to other species (1). Many studies showed that Gram-negative infections may be life-threatening, thus, it is important to identify them early and start the appropriate antimicrobial treatment as early as possible (2).
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), identify Klebsiella pneumoniae (K. pneumoniae) as a significant global concern with carbapenem resistance (3). The infections caused by hospital-acquired strains of Carbapenem-resistant K. pneumoniae (CRKP) are widespread in endemic areas. Historically, polymyxins have been used to treat CRKP infections, either alone or in combination with other antibiotics, but this has been associated with adverse effects (4). Their neurotoxicity and nephrotoxicity as well as the availability of comparably "safer" medications like β-lactams led to their discontinuation (5). By overcoming the pharmacokinetics (Pk) (low lung penetration) and toxicity (renal injury) of polymyxins, the ceftazidime-avibactam/aztreonam (CAZ/AVI/AZM) combination has made it possible to treat severe infections caused by multidrug-resistant (MDR) Gram-negative bacilli. Due to its efficacy against OXA-48 and KPC, it is also effective against CRKP. The primary mediators of carbapenem resistance in CRKP in India were found to be OXA-48 and the New Delhi Metallo-β-lactamases (NDM) (6). Ceftazidime-avibactam (CAZ/AVI) is effective against infections caused by However, it showed ineffectiveness against NDM. On the other hand, aztreonam (AZM) maintains hydrolytic stability against class B metallo-β-lactamases (MBL). The combined administration of AZM and CAZ/AVI has been documented to be efficacious in managing the infections caused by Enterobacteriaceae that produce MBL (7). The antibiotic sensitivity pattern of bacterial isolates causing CRKP infection need to be introduced to improve the treatment strategies and combat the antibiotic resistance. The study aimed to compare the rapid methods of detecting antibiotic resistance by carbapenemase enzymes with the conventional laboratory methods in diagnosing CRKP infections.
2.1 Sampling and identification
The inclusion criteria composed of these items: K. pneumoniae isolates that were identified as carbapenem-resistant using antimicrobial susceptibility testing (AST) by the Kirby Bauer disc diffusion method and VITEK®2 system, and bacterial isolates obtained from the clinical samples of in-patients. The exclusion criteria consisted of the bacterial isolates from the patients already received antibiotic therapy, and Hyper mucoid colonies of K. pneumoniae that may lead to false positive or false negative results with the Rapidec® Carba NP test.
This prospective study was conducted on the samples from January 2023 to February 2024 at the Department of Microbiology, Central Laboratory Sree Balaji Medical College and Hospital (SBMCH) Chennai, India. The K. pneumoniae was isolated in the laboratory from the clinical samples of in-patients. The clinical samples included urine, pus, wound swabs, tracheal aspirates, sputum, blood, and body fluids. The samples were inoculated on nutrient agar, 5% sheep blood agar, and MacConkey agar plates and incubated at 37°C overnight. The isolates were further identified by the standard biochemical methods followed by AST both by manual method using Kirby Bauer disc diffusion (DD) and automated method using VITEK®2 system (bioMérieux, France). The Rapidec® Carba NP test was also conducted to detect carbapenemase.
The efficacy and potential synergistic effects of the combination treatment comprising CAZ/AVI with AZM were evaluated using the E-strip gradient stacking method.
The AST was done using the following discs (Hi Media Laboratories Pvt. Ltd., Chennai, India), ampicillin (10 μg), cefazolin (30 μg), ceftriaxone (30 μg), amikacin (30 μg), gentamicin (10 μg), cefotaxime (30 μg), ceftazidime (30 μg), cefepime (30 μg), ciprofloxacin (5 μg), tobramycin (10 μg), tetracycline (30 μg), cefuroxime (30 μg), imipenem (10 μg), meropenem (10 μg), piperacillin/tazobactam (100/10 μg), amoxicillin-clavulanate (20/10 μg), co-trimoxazole (25 μg), aztreonam (30 μg), and chloramphenicol (30 μg). Resistance to carbapenems was identified by the Kirby Bauer disc diffusion (DD) method. The MIC was done by the VITEK®2 system (bioMérieux, France), and the Carba NP test was performed as per the CLSI 2023 M 100 33rd edition guideline. Broth microdilution was used for testing colistin, as knowledge of colistin MIC will help in making treatment decisions for MDR Gram-negative infection. The standard reference strain was Escherichia coli (E. cloi) (ATCC 25922) (Microbiologics Inc.).
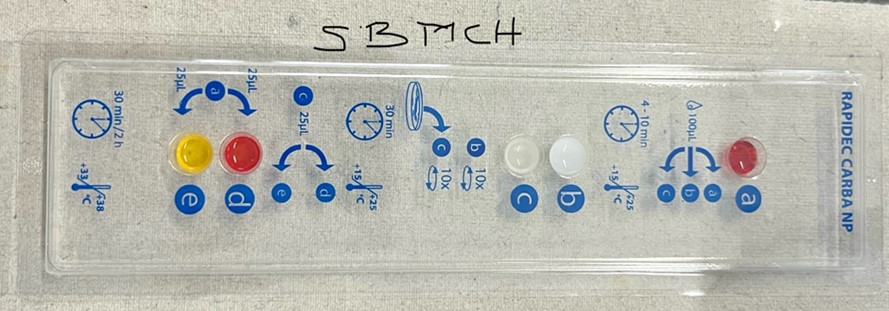
2.2 Rapidec® Carba NP test
The Rapidec® Carba NP (Carbapenemase Nordmann-Poirel) kit, developed by bioMérieux (La Balme-les-Grottes, France), was used to identify carbapenemase as per the manufacturer's instructions. The test organisms (2 ml) were added to the test strips, and incubated for 30 min. The color change was observed. The carbapenem producers changed the color to yellow, but the color remained red in those with no carbapenem production.
2.3 Detection of CAZ/AVI and AZM synergy using E-strip
AZM-containing E-test strips were placed on the Muller Hinton agar plate streaked by the lawn culture method and allowed to diffuse. After 10 min of incubation, the first E-test strip of AZM was removed. The CAZ/AVI E-strip was placed over the impression of the AZM E-strip. The AZM strip was again placed over the CAZ/AVI strip by the gradient stacking. Then, it was incubated for 16–18 hr to record the MIC values (8).
Ethical approval was obtained from the Institutional Human Ethics Committee (Ref. No. 002/SBMCH/IHEC/2024/2193).
2.4 Statistical analysis
The statistical analysis was performed using SPSS version 22 for data entry and calculation. Antibiotic susceptibility results from 120 K. pneumoniae isolates were evaluated using descriptive statistics to determine the resistance patterns, with resistance rates calculated for each antibiotic. The efficacy of the CAZ/AVI and AZM combination treatment was analyzed using synergy testing data from E-strip methods. The frequency distributions and percentages were used to summarize the resistance data, while graphical representations were used to visualize the antibiotic susceptibility patterns and treatment efficacy.
A total of 120 MDR K. pneumoniae were isolated from the clinical samples. Among them, 67 isolates were found to be carbapenem-resistant by performing AST using disc diffusion method in Muller Hinton Agar, VITEK, and Rapidec® Carba NP Methods. The wound culture samples yielded the highest number of resistant organisms, comprising 25 out of 67 isolates (37.3%). Following this, urine samples accounted for 22 out of 67 isolates (33%), while respiratory samples contributed 11 out of 67 isolates (16.4%). Pus samples had 6 out of 67 isolates (9%), and blood and tissue samples each had 1 out of 67 isolates (1.5%). Finally, Drainage Tube swabs also yielded 1 out of 67 isolates (1.5%) (Table 1). Among the 120 samples, 54 (45%) were female and 66 (55%) were male. The study population age range was 16–80 years old, with the mean age of 54.3 years old shown in Figure 1.
Table1. Number of samples obtained from various clinical specimens
| S. NO | Clinical sample | Number of clinical samples | Percentage (%) |
| 1 | Urine | 40 | 33.3 |
| 2 | Sputum | 21 | 17.5 |
| 3 | Blood for culture | 3 | 2.5 |
| 4 | Wound Swab | 36 | 30 |
| 5 | Pus | 11 | 9.1 |
| 6 | Drain Tube (DT) | 1 | 0.83 |
| 7 | Bronchoalveolar lavage fluid (BAL) | 5 | 4.1 |
| 8 | Tissue | 1 | 0.83 |
| 9 | Endotracheal Tube (ET) | 2 | 1.6 |
| Total | 120 | 100 |
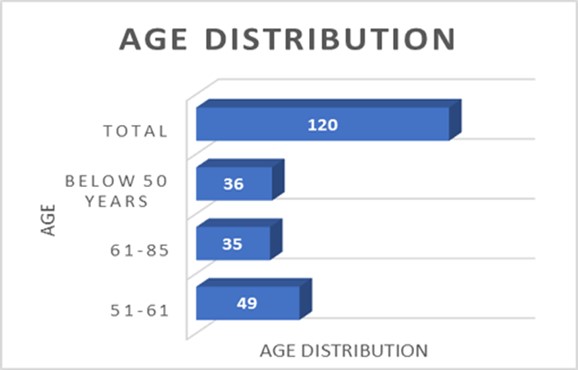
Figure1. Age distribution of the study population
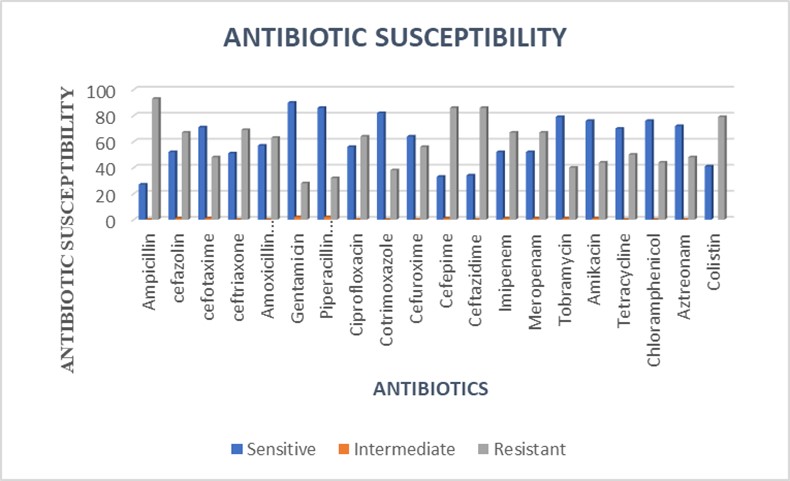
The isolates exhibited high resistance to ampicillin, cefepime, ceftazidime, imipenem, meropenem, colistin, ceftriaxone, cefazolin, and ciprofloxacin. They demonstrated the least resistance to gentamicin, cotrimoxazole, tazobactam, and piperacillin (Figure 2).
| Antibiotics | Strength (µg) | Susceptibility | Intermediate | Resistant | |||
| No. of isolates | % | No. of isolates | % | No. of isolates | % | ||
| Meropenem | 10 | 53 | 44 | - | - | 67 | 56 |
| Imipenem | 10 | 53 | 44 | - | - | 67 | 56 |
| Piperacillin/Tazobactam | 100/10 | 88 | 73 | - | - | 32 | 27 |
| Tobramycin | 10 | 80 | 67 | - | - | 40 | 33 |
| Cotrimoxazole | 1.25/23.75 | 82 | 68 | - | - | 32 | 27 |
| Gentamicin | 10 | 92 | 77 | - | 28 | 23 | |
| Amoxicillin/Clavulanic Acid | 20/10 | 57 | 48 | 1 | 0.8 | 62 | 52 |
| Ampicillin | 10 | 27 | 22 | - | - | 93 | 78 |
| Cefotaxime | 30 | 72 | 60 | - | - | 48 | 40 |
| Ceftriaxone | 30 | 69 | 58 | - | - | 51 | 43 |
| Cefuroxime | 30 | 64 | 53 | 1 | 55 | 46 | |
| Cefazolin | 30 | 53 | 44 | - | - | 67 | 56 |
| Ceftazidime | 30 | 70 | 58 | - | - | 50 | 42 |
| Ciprofloxacin | 5 | 56 | 47 | - | - | 64 | 53 |
| Amikacin | 30 | 76 | 63 | 1 | 0.8 | 43 | 36 |
| Aztreonam | 30 | 72 | 60 | 2 | 1.6 | 46 | 38 |
| Tetracycline | 30 | 70 | 58 | - | - | 50 | 42 |
| Chloramphenicol | 30 | 76 | 63 | 1 | 0.8 | 43 | 36 |
Rapid test for carbapenemase detection
The presence of carbapenemase was confirmed with the Rapidec® Carba NP test. This test is a fast and easy method to screen the isolates resistant to carbapenems, which can identify the colorimetric positive signal in less than 1 hr. The Rapidec® Carba NP test was used to identify the carbapenemase enzyme in these species, and 56% of them were shown to be carbapenemase producers by changing the color to yellow. Among the total of 67 carbapenemase-positive isolates, the Rapidec® Carba NP test detected 65 samples, resulting in a sensitivity of 97% for carbapenemase detection (Figure 3).
Treatment for the infections caused by Enterobacteriaceae containing NDM remains challenging. The AZM-AVI in vitro activity has been shown and the results are encouraging (9).
For the CRKP isolates, synergy was observed between CAZ/AVI E-strip and AZM E-strip in 66 out of 67 isolates (98.75%). Among them, 63 (95%) isolates were resistant to both CAZ/AVI and 63 (95%) were resistant to only AZM (Figure 4).
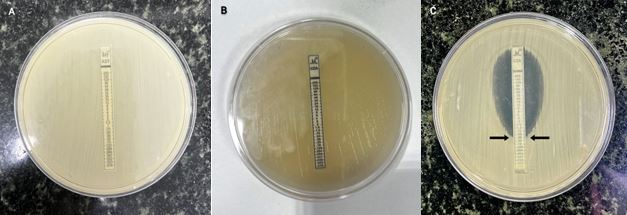
Figure 4. MIC by E-Strip gradient stacking method using AZM with CAZ/AVI E-strip. A) Isolates resistant to AZM. B) Isolates resistant to CAZ/AVI. C) Gradient strip-based susceptibility testing of AZM and CAZ/AVI. The arrow shows the MIC value.
When treating MDR pathogens and extended-spectrum β-lactamases, carbapenem is the preferred drug. More and more evidence suggest the presence of carbapenem resistance, particularly in hospital-associated infection (HAI) cases (12) as seen in a study in 2021 on CRKP that author showed high resistance to carbapenems (13).
In our study, the Rapidec® Carba NP test demonstrated a sensitivity rate of 97% for carbapenemase identification, detecting 65 out of 67 isolates that tested positive for carbapenemase. Nilgün Kansak et al., showed 97.8% sensitivity for the Rapidec® Carba NP test for the isolates tested positive for carbapenemase. Among 90 PCR-positive isolates, one was positive for OXA-48 and one was positive for OXA-48 + NDM, yet both tested negative with the Rapidec® Carba NP test (14).
In an evaluation of the Rapidec® Carba NP test, Laurent Dortet et al., showed in their study an overall best performance with 99% sensitivity and 100% specificity (15).
The Rapidec® Carba NP test is highly valuable in rapidly identifying carbapenemase-producing isolates in hospitals due to its rapid results obtained within 2 hr, and its ease of use, which requires no specialized technical expertise. This early detection will facilitate the implementation of appropriate infection control measures, leading to a notable reduction in the transmission of these infections. However, these findings highlight the effectiveness of the Rapidec® Carba NP test as a diagnostic tool for detecting carbapenemase-producing isolates. The relatively high cost of the Rapidec® Carba NP test may restrict its routine use in the clinical laboratories in resource-limited settings (16).
Further laboratory studies included in vitro synergy of CAZ/AVI and AZM against the K. pneumoniae isolate using an E-strip gradient stacking method (17). Our finding of synergy between CAZ/AVI E-strip and AZM showed significant results for the E-strip against CRKP isolates. Among the CRKP isolates tested, a remarkable 98.75% exhibited synergy when both CAZ/AVI and AZM E-strips were used together. The combined effect of CAZ/AVI and AZM in inhibiting the growth of CRKP isolates enhanced their effectiveness against MDR strains.
Our findings add to the increasing evidence supporting the use of combination therapy as a strategy to combat MDR Gram-negative infections. The high rate of synergy observed in our study suggests that CAZ/AVI and ATZ will provide a promising treatment option for the CRKP infections, particularly in the settings with limited therapeutic alternatives.
Maraki et al., evaluated in vitro synergy between AZM and CAZ/AVI against 40 strains of K. pneumoniae that produced MBL, serine β-lactamase, and MDR using an E-strip test-based synergy technique. The results showed 97.5% synergy in the CAZ/AVI-AZM combination group (18). From the clinical samples, Jayol A. et al., isolated 63 isolates of K. pneumoniae, 15 of which produced NDM-like molecules. Using E-strip test, it was identified that the combination of CAZ/AVI and AZM was efficient against K. pneumoniae that produced NDM (19).
This study has clinical implications in resource-limited settings, where polymyxin resistance is rising. The combination of CAZ/AVI and AZM provides a promising alternative, particularly for the CRKP infections with limited therapeutic options.
Resistance due to New Delhi Metallo-β-lactamase (NDM) enzymes is an important challenge in the management of CRKP infections. When combined with CAZ/AVI, AZM remains stable against NDM making it an effective treatment for the CRKP strains that produce NDM when other β-lactams are ineffective.
It is essential to mention the limitations of our study and the need for further clinical validation. This study was carried out only in our hospital and was not a multi-center study, which makes lack of genes prevalence in other locations. Furthermore, only carbapenemase production was studied as the method of resistance without including other mechanisms. The Rapidec® Carba NP test can be used as a point-of-care screening method for the CRKP in the limited-resource settings to aid in rapid diagnosis to early initiate the appropriate antibiotics treatment. Multi-centric studies will provide comprehensive data on the CRKP infections to know the trends in the antibiotic resistance.
Future research should focus on elucidating the optimal dosing regimens, treatment durations, and clinical outcomes associated with the combination therapy using CAZ/AVI and AZM in the patients with CRKP infections. Additionally, ongoing surveillance of the antibiotic resistance patterns and the emergence of novel resistance mechanisms will be crucial for the treatment strategies and ensuring the continued efficacy of the combination therapies against MDR pathogens.
In conclusion, the rapid and accurate detection of carbapenemase is vital for the immediate implementing control measures, administering appropriate antibiotics, and preventing the spread of infections among patients. The Rapidec® Carba NP test is performed as a valuable tool, offering a swift and reliable method for identifying carbapenemase-producing organisms. The Rapidec® Carba NP test provides rapid results but is cost-effective. We have identified a synergistic interaction between CAZ/AVI and AZM against these CRKP isolates. Furthermore, the identification of this synergistic effect shows the importance of exploring alternative treatment approaches to combat antibiotic resistance. Utilizing the synergistic interactions between CAZ/AVI and AZM, we can enhance the treatment efficacy while minimizing the development of further resistance.
In summary, our study provides important considerations regarding the treatment options for the CRKP infections. It highlights the ongoing need for the novel therapeutic strategies to address the antibiotic resistance effectively. This work can raise awareness among the healthcare personnel, in the treatment and diagnosis of MDR Gram-negative infections in the changing patterns of drug resistance in the CRKP infections. This calls for the stringent adherence to the antibiotic stewardship programs in curtailing the spread of drug resistance at various levels. It makes potentials for the research on MDR Gram-negative infections among diagnostic laboratories for the clinical implementation.
None.
Ethical Considerations
Ethics approval was obtained from the Institutional Human Ethics Committee (Ref. No. 002/SBMCH/IHEC/2024/2193).
Authors’ Contributions
Raveendran Praveena contributed to the study conception and design, revising for important intellectual content and final approval of the published version. Sharon Christina M. contributed to the conception and design, data acquisition, analysis, and interpretation, drafting the manuscript, and revising for important intellectual content.
No Funding.
Conflicts of Interest
Received: 2024/06/26 | Accepted: 2024/09/22 | ePublished: 2024/09/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |