BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2368-en.html


 , Louisa Ivana Utami2
, Louisa Ivana Utami2 

 , Andi Yasmon3
, Andi Yasmon3 

 , Fitri Azizah4
, Fitri Azizah4 

 , Hanny Nilasari4
, Hanny Nilasari4 

 , Inge Ade Krisanti4
, Inge Ade Krisanti4 


2- Master’s Programme in Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia & Department of Microbiology, Faculty of Medicine, Universitas Sultan Ageng Tirtayasa, Jakarta, Indonesia
3- Department of Microbiology, Faculty of Medicine, Universitas Indonesia- Cipto Mangunkusumo Hospital, Jakarta, Indonesia
4- Department of Dermato-venereology, Faculty of Medicine, Universitas Indonesia- Cipto Mangunkusumo Hospital, Jakarta, Indonesia
Neisseria gonorrhoeae (N. gonorrhoeae) is a diplococcus Gram-negative bacterial species that can infect mucous membranes of the urogenitalia, eyes, anus, and throat, with a clinical manifestation of mucopurulent discharge (1, 2). In 2017, the World Health Organization (WHO) listed gonorrhoea as the second most common sexually transmitted infection (STI) in the world (3). The WHO data in 2016 showed 376 million new cases of STIs, covering 87 million cases of gonorrhoea worldwide. This disease is transmitted mainly through the sexual contact from an infected partner with or without symptoms or direct contact with the patient’s discharge.
Antibiotic resistance of N. gonorrhoeae has been reported to almost all antibiotics used as the treatment options, including first-line antibiotics such as cephalosporin and macrolide. Failure to treat gonorrhoea with the antibiotic cefixime was first reported in Japan in 2001. The WHO's Global Antimicrobial Surveillance Project (GASP) reported a 66% resistance rate to cephalosporin antibiotic in 2014 (4). Decreased susceptibility to the third-generation cephalosporin antibiotics (cefixime and ceftriaxone) in the form of an increase in the minimum inhibitory concentration (MIC) value has been reported from Sweden, Austria, Australia, England, Japan, Bhutan, India, and Indonesia (5). The low prevalence of N. gonorrhoeae resistance to azithromycin in France was essentially due to multiple genetic mutations (6). High-level azithromycin resistance is likely to emerge rapidly in N. gonorrhoeae by a simple point mutation in the 23S rRNA that was reported in the United Kingdom (7).
In Indonesia, the epidemiology of gonorrhoea cases has not been recorded properly. Although, the reports are limited, several studies have been done on the susceptibility pattern of N. gonorrhoeae in Indonesia. In a study from 2017, resistance to cefixime was 25% in high-risk groups in Surabaya (8). Puspandari et al. (9) reported a 3% resistance to azithromycin.
Several studies have reported an emerging molecular resistance of N. gonorrhoeae, such as the acquisition of mosaic and non-mosaic penA alleles, with or without substitutions at amino acid position 501 of the encoded penicillin-binding protein 2 (PBP2), and penA Gly545Ser has been associated with decreased susceptibility to ESCs (10, 11). Thus, mutation in 23S rRNA, such as A2059G and C2611T mutations, correlates with azithromycin (AZM) resistance in N. gonorrhoeae. A single base substitution can result in high resistance in N. gonorrhoeae (12, 13). Due to the limited choice of antibiotics, along with the rapidly increasing resistance of N. gonorrhoeae to antibiotics, evaluating the susceptibility patterns and molecular characteristics of MDR N. gonorrhoeae is important to determine the appropriate choice of antibiotics.
This cross-sectional study was conducted in Jakarta, Indonesia. The specimens were collected using consecutive sampling and examined in laboratory. The study was approved by the Ethical Committee of Medicine Faculty, Universitas Indonesia, under the number KET-667/UN2.F1/ETIK/PPM.00.02/2020, and all patients provided written consent prior to the enrolment.
Clinical specimens
endocervical specimens (41 samples) were collected from female sex workers in Pasar Rebo Rehabilitation Center and streaked onto the chocolate agar and then gently rolled onto glass objects. The specimens were fixed by passing on flame, and placed into a candle jar at room temperature (25-30°C). The remaining swab was placed into a cryotube containing 1 mL PBS and stored in an ice box. Subsequently, all specimens were transported to the Clinical Microbiology Laboratory Faculty of Medicine Universitas Indonesia (CML FMUI). The glass slides were exposed to Gram staining, and the inoculated specimens on the chocolate agar medium were placed into a CO2 incubator. The swab was centrifuged at 12,000 RPM for 10 min, and the supernatant was removed and stored at -80°C until molecular testing.
Bacteria identification
Gram staining was conducted at CML FMUI according to the manufacturer’s instructions (BD BBL, USA). The crystal violet was used as primary stain, iodine solution for mordant, acetone and isopropanol as decolourizer, and safranin for counterstaining. The intracellular Gram-negative kidney-shaped diplococci in polymorphonuclear leukocytes observed under the oil immersion (1,000x magnification) wasthe characteristic for the presumptive diagnosis of gonorrhoea. The culture specimens on the chocolate agar were placed into a CO2 incubator at 35–37°C for 24–48 hr. The suspected colony was identified by Gram staining, oxidase testing, and biochemical identification using the VITEK® 2 NH card (BioMérieux, France). The N. gonorrhoeae isolates were tested for the susceptibility and stored in tryptic soy broth (TSB) and 10% glycerol at -20°C for the molecular testing. For the control strain of N. gonorrhoeae, the ATCC 43069 strain was used.
Susceptibility testing for N. gonorrhoeae
The antimicrobial susceptibility of N. gonorrhoeae was assessed using the disk diffusion method for the susceptibility testing to penicillin, cefixime, kanamycin, ciprofloxacin, and azithromycin. The E-test was used for susceptibility to ceftriaxone, levofloxacin, and tetracycline. The inhibition zone diameters and the MIC values were measured. Based on the break point of each antibiotic on CLSI 2019 the strains were categorized as susceptible, intermediate, or resistant.
Molecular assay to detect MDR N. gonorrhoeae
DNA extraction was carried out with a DNA mini kit (Qiagen) according to the instructions (14). Detection of N. gonorrhoeae was carried out using the SYBR green real-time PCR (15). Each reaction included positive and negative controls. The melting curve was analyzed after amplification. Primers specific to the opa gene were reported by Geraats-Peters et al. (16). Multiplex real-time PCR with high-resolution melting (HRM) was used to analyze gene mutations in penA and 23S rRNA, correlating to the resistance against extended-spectrum cephalosporins (ESCs) and azithromycin (AZM) (14, 17, 18). The thermal cycling was as follow: enzyme activation at 95oC for 3 min, 30 cycles of denaturation at 95oC for 10 sec, annealing at 62°C for 10 sec, and extension at 72°C for 10 sec. The high-resolution melting (HRM) analysis was performed as follow: 10 sec at 95°C and 1 min at 60°C; subsequently, the sample was heated to 95°C with a ramping time of 0.025°C/sec to obtain a florescent signal. The primers specifications are mentioned in Table 1. Synthetic DNA was designed as reference for both mutant penA and 23S rRNA in multiplex PCR.
DNA sequencing to confirm penA and 23S rRNA mutations
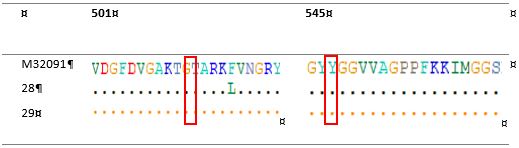
The nucleotide sequences of the penA Ala501, Gly545Ser, 23S rRNA A2059G and C2611T genes were confirmed using the Sanger method. The sequencing results in the form of nucleotide sequences accompanied by the electrographs were analyzed by BioEdit software to ensure that the peaks of each nucleotide base were high and separated from each other. The primers for PCR and DNA sequencing are listed in Table 2. The DNA sequencing was performed using one forward primer from penA gene and one reverse primer from 23S rRNA gene. The DNA sequence was compared with that of wild-type N. gonorrhoeae to confirm the sequences of penA Gly545Ser, penA Ala501, 23S rRNA positions A2059G and C2611T. The N. gonorrhoeae M32091 and X672931 were used as reference strains.
Table 1. Primers to detect mutations in penA and 23S rRNA of N. gonorrhoeae.
| Target | Primer | Amplicon length | Multiplex type |
| penA Ala501 | F-CCCGCCCCCGCCGTCGGCGCAAAAACCGGTACG | 79 | Duplex 1 |
| R-CCCGCCCCCGCCAATCGACGTAACGACCGTTAACCAACTTACG | |||
| 23S rRNA C2611T | F-ACGTCGTGAGACAGTTTGGTC | 67 | Duplex 1 |
| R-CAAACTTCCAACGCCACTGC | |||
| penA Gly545Ser | F-CCCGCCCCCGCCGACTGCAAACGGTTACTA | 142 | Duplex 2 |
| R-CCCGCCCCCGCGGCCCCTGCCACTACACC | |||
| 23S rRNA A2059G | F-CTACCCGCTGCTAGACGGA | 49 | Duplex 2 |
| R-CAAACTTCCAACGCCACTGC |
Table 2. Primer for sequensing penA and 23S rRNA genes in N. gonorrhoeae
| Gen | Primer | Amplicon length |
| penA |
|
348 bp |
| 23S rRNA |
|
|
The presumptive diagnosis of gonorrhoea using the microscopic examination of Gram-stained endocervical specimens was based on the presence of intracellular Gram-negative kidney-shaped diplococci in polymorphonuclear leukocytes. From the 41 specimens, 9 were Gram-negative, and 14 were successfully identified, but only 9 isolates could be used for the susceptibility testing. The antimicrobial susceptibility testing (AST) showed that all tested N. gonorrhoeae isolates (100%) were resistant to penicillin, tetracycline, and ciprofloxacin. Resistance to kanamycin, levofloxacin, and cefixime was found in 77.8%, 33.3% and 11.1% of the isolates, respectively. No isolate was resistant to ceftriaxone and azithromycin. Details of the antibiotic resistance test results can be seen in Table 3.
Tabel 3. AST results of N. gonorrhoeae
| Group | Antibiotics | Result of antibiotic susceptibility (n=9) | ||
| Sensitive (%) | Intermediate (%) | Resistant (%) | ||
| β-lactam | Penicilin | - | - | 100 |
| Cephalosporin | Cefixime | 88.9 | - | 11.1 |
| Ceftriaxone | 100 | - | - | |
| Aminoglycosides | Kanamycin | 11.1 | 11,1 | 77.8 |
| Fluoroquinolone | Ciprofloxacin | - | - | 100 |
| Levofloxacin | 66.7 | - | 33.3 | |
| Macrolide | Azithromycin | 100 | - | - |
| Tetracycline | Tetracycline | - | - | 100 |
Detection of N. gonorrhoeae from direct specimens using SYBR green real-time PCR molecular test showed 15% increase in positivity compared to culturing (49% vs 34%). Compared to the Gram staining, the increase in positive results using SYBR green real-time PCR was 27% (49% vs 22%).
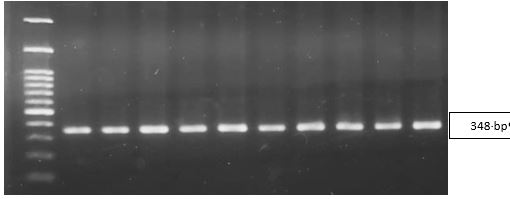
The N. gonorrhoeae-positive endocervical specimens were subjected to multiplex real-time PCR. The positive control used in the detection of penA and 23S rRNA resistance genes came from synthetic DNA containing the penA gene mutations Ala501, Gly545Ser, 23S rRNA A2059G and C2611T of N. gonorrhoeae. Figure 1 shows the results of optimising the penA rRNA gene PCR products using the electrophoresis. The BLAST results showed the amplification of 348 bp product from penA. Detection of duplex resistance genes 1 and 2 in the clinical specimens can be seen in Figure 2.
The amino acid sequence corresponding to the reference wild-type M32091 N. gonorrhoeae can be seen in Figure 3.
Sequencing of the 781-bp amplified product of the 23S rRNA gene for all specimens did not show any mutation in the 23S rRNA A2059G and C2611T genes. This result was in accordance with the findings of the SYBR green real-time PCR.
Figure 1. Optimizing results of penA gene PCR products. Line 1: M: marker 100-1200 bp, Lines 2-11: specimens.
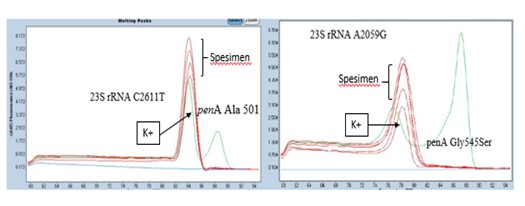
Figure 2. Detection of duplex 1 and 2 resistance genes in the clinical specimens. For the 23S rRNA gene: no Tm value was found that was the same as the positive control. For the penA gene: no mutation curve was found like the positive control. Positive control: synthetic DNA containing the resistant gene.
Figure 3. Allignment of amino acid sequences of penicillin-binding protein 2 (PBP2) penA Ala501 and penA Gly545Ser
In this study, 14 (34%) of the 41 specimens were grown successfully on the chocolate agar culture medium. The identification test of the colonies using Gram staining showed Gram-negative diplococcal colonies, according to the morphology of N. gonorrhoeae. The oxidase test carried out on 14 isolates resulted in a purple colour, according to the description of oxidase-producing bacteria. Colony purification was carried out for identification using an assimilation test with the VITEK2® NH kit. As many as 6 of the 14 isolates could not grow again. Identification tests with the VITEK2® NH kit were carried out on 8 isolates that were successfully subcultured. Based on the results, 75% of the 8 isolates were identified as N. gonorrhoeae, whereas one isolate was identified as N. meningitidis, and one was unidentified.
Soloaga et al. (19) reported that the identification test using VITEK had a sensitivity of 96.5% and a specificity of 100% in the pure cultures of N. gonorrhoeae. The culture of urogenital specimens has a sensitivity of 85%-95% under optimal conditions and a specificity of 100%, indicating that it can correctly identify N. gonorrhoeae (20).
The results of this study and several reports on the sensitivity and specificity of the culture method for the identification of N. gonorrhoeae are promising, but N. gonorrhoeae isolates are difficult to grow in vitro. The intrinsic factor of N. gonorrhoeae is strongly influenced by the external factors, which must be fulfilled to obtain the optimal growth in medium. Unemo and Shafer (13) and Ng and Martin (21) reported that the successful cultur of N. gonorrhoeae is also influenced by the quality of the specimen at the pre-analytical stage, including the selection of the specimen collection location, the collection method, and the transportation conditions to the laboratory. It is also impacted by the problems found at the analytical stage related to the complex composition of the growth medium, and the requirement for the CO2, and the limited options for the confirmatory testing.
The SYBR green real-time PCR targeting the opa gene successfully detected 49% N. gonorrhoeae from the swab samples.This finding was consistent with the results reported by Dona et al. (14), according to whom the sensitivity of SYBR green real-time PCR targeting the opa gene had a positivity rate of 100%. These results are promising regarding the difficulties in obtaining pure isolates from the specimens due to failure to culture N. gonorrhoeae. Molecular testing can help overcome the problems of culturing N. gonorrhoeae such as contamination of the normal flora in the genitalia, the small number of specimens, and previous exposure to antibiotics. In addition, the molecular detection method is faster than the culture method.This is in line with the findings of Yasmon et al. (15), who succeeded in detecting N. gonorrhoeae directly from the clinical specimens, with sensitivity and specificity values of 100%. The AST showed that all N. gonorrhoeae isolates were resistant to penicillin and tetracycline. Unemo and Shafer (13) and Tapsall et al. (22) reported that penicillin resistance has been occurring since 1976 and has spread globally through the plasmids and chromosomes. These results were in line with previous findings reported by Wi et al. (3), Unemo and Shafer (13) and Mabonga et al. (23), stating that the antibiotics penicillin and tetracycline are not recommended as treatment options for the gonorrhoea due to the high resistance rates globally.
The resistance of N. gonorrhoeae to the fluoroquinolone class of antibiotics (levofloxacin) in this study was 33.3%, and all isolates showed resistance to ciprofloxacin. These results were similar to those reports from Uganda, where cases of ciprofloxacin resistance were found in all isolates (23). Shigemura et al. (24) reported 56.5% N. gonorrhoeae resistance to levofloxacin in Japan. The resistance of N. gonorrhoeae to kanamycin was 88.9%, which was lower than the 93% reported by Apalata et al. (25). Puspandari et al. (9) using the FSW isolates in Jakarta, Tangerang, and Palembang, reported 29.6% resistance to kanamycin. These results indicate an increase in N. gonorrhoeae resistance to kanamycin over time, justifying the stop of the use of this antibiotic as the drug of choice for gonorrhoea infections.
The WHO and Indonesian Ministry of Health guidelines for the treatment of gonorrhoea infections recommend the use of cefixime and azithromycin (4, 26, 27). The resistance test for cefixime in this study showed that 11.1% of the N. gonorrhoeae isolates were resistant to cefixime. Resistance started to be discovered from the antibiotic cefixime, which carries the risk of treatment failure, as reported in Japan and several countries in Europe, Canada and South America (28-30). The sensitivity of N. gonorrhoeae to azithromycin found in this study was still high at 100%. In this study, the sensitivity to the antibiotic ceftriaxone, which is still recommended for the treatment of gonorrhoea infections, was 100%. Puspandari et al. (9) reported 2.8% N. gonorrhoeae resistance to azithromycin. Similarly, for Zimbabwe, Latif et al. (12) showed that N. gonorrhoeae was still sensitive to azithromycin and ceftriaxone.
In this study, 11.1% of the isolates were found resistant to cefixime, which could have been caused by the mutations in the penA gene or the mosaic penA allele, which encodes PBP2 (5, 28, 30). However, SYBR green real-time PCR did not show any amplification of the penA Ala501Val/Pro and Gly545Ser genes. The negative amplification for both penA Ala501 and Gly545Ser means that other factors may be responsible, such as mutation in the mtrR promoter, which resulted in overexpression of the mtrCDE efflux pump, or penB mutation, which is a mutation determinant of cefixime (10). Ochiai et al. (28) reported that changes in penA with three amino acid substitutions in the PBP2 mosaic can reduce the sensitivity of N. gonorrhoeae to cefixime and that the mosaic structure of penA is associated with the genetic polymorphisms in mtrR, porB1b and ponA.
Mutations in 23S rRNA C2611 and A2059, associated with AZM resistance, were not observed in this study, which is in agreement with the AST results. In a previous study, 23S rRNA mutations were acquired in at least 3 from 4 alleles of N. gonorrhoeae (10). Other resistance mechanisms, such as enzymatic resistance through rRNA methylase, which causes the blocking of azithromycin binding to 23S rRNA on position 2058, are also possible resistance mechanisms of N. gonorrhoeae to azithromycin (31). Another mechanism of azithromycin resistance involves mutations in mtrR, causing the overexpression of efflux pumps. The mutations can occur in the mtrR promoter region in the form of adenine deletion or the mtrR repressor A39T/G45D, as found in studies in America and Portugal (32-34). In a study by Shigemura et al. (24) in Japan, mutations in the mtrR promoter region caused an increase in the MIC values (> 0.5 mcg/mL).
In the present study, N. gonorrhoeae could be detected in the endocervical samples with higher sensitivity by SYBR green real-time PCR. The susceptibility of N. gonorrhoeae to cefixime, ceftriaxone, and azithromycin found in this study was above 80%. Decreased susceptibility was found to fluroquinolone; 33.3% of the isolates were resistant to levofloxacin, and all isolates showed resistance to ciprofloxacin.
Penicillin, tetracycline, and kanamycin are no longer recommended as the drugs of choice for gonorrhoea infections due to the high resistance rates globally. Decreased susceptibility to cefixime as one of the drugs of choice for gonorrhoea treatment in Indonesia was also found. The molecular detection of cefixime resistance by PCR does not correlate with penA Ala501 and Gly545Ser mutations. Other genes may be responsible, such as mutations in the mtrR promoter, resulting in the overexpression of the mtrCDE efflux pump, or penB mutations, determining cefixime resistance. All isolates were susceptible to azithromycin, which was in line with its molecular characteristics.
The strength points of this study are to show the resistance pattern of N. gonorrhoeae to the various antibiotics in Indonesia, and the SYBR green real-time PCR that successfully detected N. gonorrhoeae from endocervical swabs, so that it can help overcome various obstacles of the culture method and susceptibility testing of N. gonorrhoeae. In addition, it was found that decreased susceptibility to cefixime as one of the drugs of choice for the treatment of gonorrhea in Indonesia, did not correlate with penA gene mutations. The weakness of this study is that no N. gonorrhoeae isolates resistant to azithromycin were found to search for the mutations in 23S rRNA C2611 and A2059 that are associated with AZM resistance. The existing limitations are difficulty in subculturing N.gonorrhoeae isolates to grow and the limited number of clinical specimens.
Cefixime, ceftriaxone, and azithromycin can be used as drugs of choice for the treatment of gonorrhea in Indonesia. The real-time PCR from direct specimens can detect N. gonorrhoeae with a higher positivity rate compared to the culture method. Multiplex real-time PCR with high-resolution melting (HRM) can be used to analyze gene mutations of N. gonorrhoeae resistant to antibiotics.
Authors thank to Balai Rehabilitasi Pasar Rebo for all assistance and cooperation in this study. Our deepest appreciation is extended to our dedicated team from Department of Microbiology, Faculty of Medicine, Universitas Sultan Ageng Tirtayasa, Department of Microbiology and Department of Dermato-venereology, Faculty of Medicine Universitas Indonesia- Cipto Mangunkusumo Hospital, Jakarta.
Ethical Considerations
The study was approved by the Ethical Committee of Medicine Faculty, Universitas Indonesia, under the number KET-667/UN2.F1/ETIK/PPM.00.02/2020, and all patients provided written consent prior to the enrolment.
Authors’ Contributions
Y.R. designed the study. A.Y. designed the primer. L.I.U. and F.A. collected the samples. Y.R. and L.I.U. performed all statical analyses, wrote the manuscript. All authors reviewed, edited, and approved the final draft.
This study was funded by Hibah PUTI Saintekes 2020, Faculty of Medicine, Universitas Indonesia with contract number NKB-2262/UN2.RST/HKP.05.00/2020.
Conflicts of Interest
Received: 2024/05/29 | Accepted: 2024/08/25 | ePublished: 2024/09/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |