BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2221-en.html

 , Jawhar Gharbi2
, Jawhar Gharbi2 

 , Khaled Al Ahmed3
, Khaled Al Ahmed3 
 , Ali Mohamed Ali1
, Ali Mohamed Ali1 
 , Manel Ben M'hadheb4
, Manel Ben M'hadheb4 


2- Department of Biological Sciences, College of Science, King Faisal University, Al-Ahsa, Saudi Arabia ,
3- Department of Polyclinic, King Faisal University, Al-Ahsa, Saudi Arabia
4- Virology and Antiviral Strategies Research Unit UR17ES30, Higher Institute of Biotechnology, University of Monastir, Monastir, Tunisia
Respiratory viral infections are associated with a wide range of acute syndromes and infectious disease processes in children and adults worldwide. Many viruses are implicated in these infections, and these viruses are spread largely via respiratory means between humans but occasionally from animals to humans. Acute respiratory infections (ARI) are among the most common infections reported worldwide. In the 2013 global disease burden study sponsored by the World Health Organization, respiratory infections were listed as the leading cause of infectious disease and as being responsible for approximately 120 million disability-adjusted life years. Lower respiratory tract infections accounted for greater than 90%, with approximately 35% of cases occurring in children less than 5 years old. The impact of respiratory infections on human health is reflected in the large number of hospital and emergency room visits for both adults and children (e.g., in the United States, there are 140,000 to 710,000 FLU-related hospitalizations per year), where respiratory viral infection is the most common reason to seek medical care.
Influenza and pneumonia were identified as the eighth leading cause of death in the USA in 2018 (1). In Korea, viruses represent 63% of ARI; most of these viral respiratory infections belong to children younger than ten years (2). Turunen et al., (3) indicated that all children younger than two years infected with wheezing for the first time had one virus at least. Moreover, ARI was associated with viruses more than bacteria among young children (4). In Riyadh region (Saudi Arabia), 60% of ARIs were caused by viruses among hospitalized young children; 81% had lower respiratory tract infections (5). Fever, cough, sneezing, rhinorrhea, headache, and myalgia are the main symptoms of viral respiratory infections. Some viruses, including hRV and hPIV, cause exacerbations of chronic diseases such as asthma and chronic obstructive pulmonary disease (COPD); moreover, hRV may cause otitis and sinusitis (6, 7). Sometimes, gastrointestinal symptoms such as vomiting, diarrhea, and abdominal pain accompany respiratory virus infections. The reason may be due to the correlation between bronchial-associated lymphoid tissue and gut-associated lymphoid tissue (8).
Respiratory viruses are transmitted primarily through two mechanisms: (i) inhalation of infectious droplets and (ii) contact with contaminated fomites. Aerosol transmission is the most common route of infection. Large (10 to 100 µm in diameter) aerosolized droplets can transmit viruses from the index case to a new host nearby, while small (<10 µm in diameter) aerosolized droplets, produced during coughing or sneezing or through aerosol-generating procedures, can carry viral particles to new hosts several meters away. Transmission via fomites from self-inoculation of the respiratory tract mucosa is the second most common route of infection (9). Relative humidity of 20-35%, low temperature of 5-20°C, rainfall, and lousy ventilation may play a role in the transmission of respiratory viruses. However, the circulation of respiratory viruses decreases significantly at 30°C (10).
Viruses can enter a cell host by binding to some appropriate receptors. For example, sialic acid works as a receptor for hEV (11), and integrin works as a primary receptor for hAdV with the absence or low of coxsackie and adenovirus receptor (CAR) (12). Moreover, animals work as natural reservoirs for viruses; thus, some viruses can be transmitted from animal to human (Swine and Avian Influenza viruses, Coronaviruses). Fortunately, in most cases, Hemagglutinin (HA) in human Influenza viruses binds to sialic acids linked to galactose by α 2,6-linkage. In contrast, avian Influenza viruses bind to sialic acids linked to galactose by α 2,3-linkage (13). The emergence of the A/H1N1 2009 pandemic, for example, was because pigs possessed both receptors of avian and human viruses (14). Bats contain the most significant proportion of zoonotic viruses, such as SARS-CoV-1, SARS-Co-V-2, and MERS-CoV, compared with other mammals (15).
Viral isolation in cell culture, antigen detection, and molecular methods are the main techniques for respiratory virus detection. Direct and indirect immuno-fluorescence staining methods are used for antigen detection by using fluorophore-conjugated antibodies (16). Likewise, enzyme-linked immunosorbent assay (ELISA) is used to detect antigens or antibodies (17). Conventional PCR and real-time PCR are the most common molecular detection. Noteworthy, molecular detection of respiratory viruses gives satisfactory outcomes, unlike conventional methods such as viral isolation and direct immunofluorescence staining (18). This study aims at molecular detection and epidemiology of common respiratory viruses; hRSV-A, hRV, INFA, INFB, hAdV, hEV, hPIV3, and hBoV, among children in Al-Ahsa province in Saudi Arabia.
Collection of Specimens
From 30 December 2021 to 10 June 2022. Eighty-three oropharyngeal specimens with clinical symptoms were collected from children aged between 1 and 13 years consulting for ARI in the pediatric clinic of the polyclinic of King Faisal University in Al-Ahsa, Saudi Arabia. Under the supervision of the pediatrician, oropharyngeal specimens were collected from patients with the consent of their parents. The ethical approval was obtained from the ethics committee of the Deanship of Scientific Research of King Faisal University (Agreement KFU-REC/2020-10-13).
Extraction of Total Nucleic Acids (DNA/ RNA)
According to the manufacturer's instructions, DNA was extracted by DNAbler Extraction Kit (Haven Scientific, Saudi Arabia). RNA was extracted by Trizol reagent (Invitrogen/Sigma, USA) and RNAbler Extraction Kit (Haven Scientific, Saudi Arabia). The measure of extracted DNA and RNA purity was applied by a spectrophotometer, while DNA and RNA quality was applied by 1% agarose gel electrophoresis (ThermoFisher, USA). In each step of the Multiplex PCR reaction (viral genome extraction, Reverse Transcription, and PCR), we used a negative control using DEPC water and a positive control representing prototypes of RNA and DNA viruses.
Synthesis of cDNA by Reverse Transcription
In reverse transcription reaction, 10 µL of GoScript™ Reverse Transcription Mix that includes 2 µL of dNTPs, 2 µL of buffer (MgCl2 + DTT), 0.5 µL of GoScript Enzyme mix (M-MLV reverse transcriptase and recombinant RNasin ribonuclease inhibitor), 2 µL of Oligo (dT) primer, and 3.5 µL of DEPC water (Promega, USA) was added to 10 µL of RNA to synthesize cDNA (19-22). Tubes were applied to GeneAmp PCR system 9700 (Applied Biosystems) under the following program with a cycle for all steps; Primer annealing 37°C for 5 min, extension 42°C for 60 min, and inactivation enzyme 70°C for 15 min (19-22).
Detection of respiratory viruses using multiplex PCR reaction
Isolation of targeted respiratory viruses was conducted by adding 40 µL of Go Taq PCR Mix that includes 2 µL of dNTPs, 0.2 µL of Go Taq polymerase, 4 µL of mix primers (Table 1), 4 µL of buffer, and 29.8 µL of DEPC water (Promega, USA) to 10 µL of cDNA or 10 µL of DNA. Positive control and negative control were tested. Amplification was run on the following program by using GeneAmp PCR system 9700 (Applied Biosystems); initial denaturation 94°C for 3 min (a cycle), denaturation 94°C for 30 seconds (35 cycles), primers annealing 45°C for 30 seconds (35 cycles), extension 72°C for 1 min (35 cycles), and final extension 72°C for 7 min (19-22). The PCR products were stained with ethidium bromide (Sigma, USA) and separated into 2% agarose gel (ThermoFisher, USA).
Table 1. Primers used in multiplex-PCR reaction.
| Virus | Primer (F/R)* | Sequence (5’-->3’) | Reference & Target gene* | Length | |
| hAdV | AV3-F | ATG TGG AAT CAG GCT GTT GAC AG | Hexon (21) | 458 bp | |
| AV5-R | CGG TGG TGT TTT AAT GGT TTT ACT TTG TCC AT | ||||
| hBoV | Boca-F | TGG GCC ATT TAA TCC ACT TGA | VP1 (29) | 63 bp | |
| Boca-R | AAT TGA GCA GCG CGA TCAG | ||||
| INFA | INFA-F | ACA CTG ACA CAC TCT GTC AA | HA (24) | 837 bp | |
| INFA-R | ACA CTC TCC TAT TGT GAC TGGG | ||||
| INFB | INFB-F | CAC AAC AAA ACA GGA GGC | NP (24) | 1017 bp | |
| INFB-R | CAG GAT TCT TCT TAC AGC TTG | ||||
| hPIV-3 | PIV-F | TGG GGG TCA GAA GGA AG | NP (31) | 273 bp | |
| PIV-R | TAA TAT GAC AGA TGA CAC AATG | ||||
| hRSV-A | RSV-F | AGA AGT GGC TCC AGA ATA TAGG | NP (27) | 577 bp | |
| RSV-R | CTC CCA TTA TGC CTA GAC CTGC | ||||
| hEV | EV-F | AAG CAC TTC TGT TTC CCC GG | 5’-NTR (29) | 156 bp | |
| EV-R | ATT GTC ACC ATA AGC AGC CA | ||||
| hRV | OL27-F | AGG ACA CCC AAA GTAG | 5’-NTR (32) | 380 bp | |
| OL26-R | GCA CTT CTG TTT CCCC | ||||
*Abbreviation: F: Forward, R: Reverse, VP: Viral Protein, HA: Hemagglutinin, NP: Nucleoprotein, NTR: Non-translated Region.
Student's t-test was used to establish statistical significance results (P<0.05) using GraphPad Prism 6 Version 6.02.
From December 2021 to June 2022, a total of 83 samples collected from children infected with ARI were tested. The age of patients ranges from one year to thirteen years. Among them, 38 were male (45.78%), and 45 were female (54.22%). Clinical symptoms were collected from the polyclinic exam sheet. Respiratory viruses were detected in 58 samples (69.88%). Twenty-one samples (25.3%) present a co-infection. Of these, 28/58 (48.28%) were male, and 30/58 (51.72%) were female. The highest percentage of infection was for hRV (31.33%) in 26 samples, followed by hRSV-A (24.1%) in 20 samples, hEV (18.1%) in 15 samples, hPIV3 (13.25%) in 11 samples, and hAdV (8.43%) in 7 samples (Figure 1). Noteworthy, no INFA, INFB, or BoV were detected in this study.
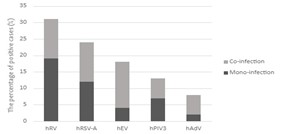
Figure 1. Percentage of mono and co-infection among analyzed samples.
Children ≤ 5 years represented 50% of positive samples. hRSV-A, hEV, and hPIV3 significantly infected children ≤ 5 years by 75%, 53.33%, and 54.55%, respectively. In contrast, children aged 10-13 years were the least infected by them. hRV significantly infected Children ≤ 5 years, followed by children aged 10-13 years in relative proportions of 42.31% and 38.46%, respectively (Table 2).
Table 2. Distribution of respiratory viruses according to age group.
| Samples, n | Age group, n (%) | Total, n | ||
| ≤ 5 years | 6-9 years | 10-13 years | ||
| All samples | 41 (49.4%) | 23 (27.71%) | 19 (22.89%) | 83 |
| Positive samples | 29 (50%) | 13 (22.41%) | 16 (27.59%) | 58 |
| Samples +hRV | 11 (42.31%) | 5 (19.23%) | 10 (38.46%) | 26 |
| Samples +hRSV-A | 15 (75%) | 4 (20%) | 1 (5%) | 20 |
| Samples +hEV | 8 (53.33%) | 4 (26.67%) | 3 (20%) | 15 |
| Samples +hPIV3 | 6 (54.55%) | 3 (27.27%) | 2 (18.18%) | 11 |
| Samples +hAdV | 2 (28.57%) | 2 (28.57%) | 3 (42.86%) | 7 |
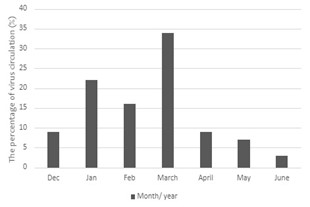
The highest percentage of positive samples was in March, with 34.48% (Figure 2). Most hRV, hPIV3, and hAdV infections were in March. In contrast, hRSV-A and hEV infections peaked in January (Figure 3).
Figure 2. Seasonality of the circulation of respiratory viruses among children.

Figure 3. Monthly circulation of the different respiratory viruses among children.
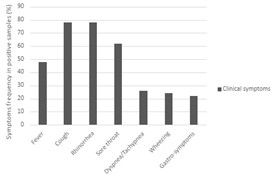
Rhinorrhea and cough were the most frequent symptoms, with 77.59%. Sore throat also represented a high percentage of 62.07% (Figure 4). However, one patient only had otitis media, and the other had an itch in the ears. hRSV-A and hEV patients had more fever than other patients, and hRV and hPIV3 had cough more than others, with 88% and 82%, respectively. hRSV-A patients infected with gastrointestinal symptoms (vomiting, nausea, and diarrhea) with 40%. Noteworthy, 100% of hRV patients had rhinorrhea. Additionally, 55% of hPIV3 patients had dyspnea or tachypnea (Figure 5).
Figure 4. Percentage of clinical symptoms of patients with positive samples.
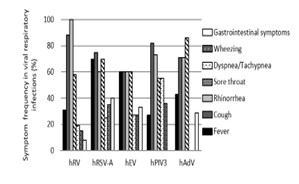
Figure 5. Prevalence of clinical symptoms associated to viral respiratory infections.
The present study is the first epidemiology study of respiratory viruses conducted in the Al-Ahsa region in Saudi Arabia. Our results indicated that 69.88% of samples were positive. This percentage is close to a previous study conducted in southwestern Saudi Arabia, where Al-Ayed et al., (4) found that 74% of patients had one virus at least. In Riyadh region (Saudi Arabia), 60% and 61% of ARI were viral infections during the 2008-2009 and 2012-2013 seasons, respectively (5, 23). Moreover, high viral respiratory infections (91.6%) were registered in Riyadh, Saudi Arabia (24). Our study and these studies gave good and close proportion because they detected viruses by molecular methods.
The rate of co-infection was 2.4% in India (19), 7-11% in Saudi Arabia (4, 5, 23), 9% in Oman (20), 12.4% in Vietnam (21), 18.4% in Brazil (22), and 15-18% in Malaysia (25, 26). Notably, co-infection in this study had a higher rate (25.3%). Noteworthy, hEV was mainly detected as a co-infection (80%). Similarly, a Brazilian study detected hEV as a co-infection (81%) (22). In previous studies, positive samples were detected in males more slightly than in females (23-25). In contrast, our study indicated that viral respiratory infections increased slightly in females than in males, in line with the report from India (19).
Human RV was predominant in the current study (31.33%). Similar to previous studies, hRV was predominant, with a percentage ranging from 25 to 33% (19, 21, 22, 24). However, a study conducted in Oman reported a low percentage of hRV (5%) (20). In the present study, hRSV-A was the second most predominant (24.1%). hRSV was also prevalent among children in the Middle East and North Africa (MENA region), with 24.4%, 63% of which corresponded to RSV-A (27). Moreover, 27% of pediatric patients had hRSV; 21% were RSV-A, while 6% were RSV-B (28). Previous studies also indicated that hRSV represented 22-24% of ARI; RSV-A was the most frequent, with about 60-90% of hRSV infections (4, 5, 23).
On the other hand, hEV, hPIV3, and hAdV were less frequent, with 18.1%, 13.25%, and 8.43%, respectively. Our finding was consistent with other studies, which recorded less frequency of hEV (2-9%), hPIV3 (1-11%), and hAdV (6-15%) (22-24, 29). Moreover, hAdV only represented 5.7% of ARI in China (30). Notably, among hPIVs, hPIV3 was the most common (2.1%), followed by hPIV1 (1.2%), hPIV2 (0.4%), and hPIV4 (0.2%), respectively (31). Furthermore, Amer et al., (5) indicated that hPIV3 was the most frequent among hPIVs, followed by hPIV2, while hPIV1 and hPIV4 were not detected in any case. No hBoV was detected in our study; this is similar to a previous study conducted in Najran, Saudi Arabia, which detected only one case of hBoV among 135 patients (4), so the absence of hBoV is not strange. On the other hand, the absence of INFA and INFB may be due to precautionary measures, where the collection of samples was during the period when the use of masks and social distancing was compulsory in Saudi Arabia but without a curfew. Additionally, this absence may be due to the prevalence of Influenza vaccination.
The most positive samples belonged to children aged from one year to 5 years which aligned with a previous study (24). Another study suggested that the second most hRV patients were children aged 1-5 years in the 2009-2010 season after infants (32). Likewise, children aged 0-4 had the highest percentage of hRV infections (33). These results of previous studies are consistent with our study. On the other hand, our results reported that hRSV-A decreased with age, in line with reports from Malaysia (26) and Qatar (34). Hellferscee et al. (35) indicated that hEV infected children aged 1-4 years more than children <1, while children aged 5-18 recorded the least rate. The present study reported a higher proportion of hEV infections in children aged 1-5 years. Noteworthy, the number of hPIV3 after a year of age decreased with age in cell culture (36) and real-time RT-PCR (31) for children ≤ 14, additionally in the multiplex RT-PCR in our study. In China, the rate of hAdV infections was different among age groups (30), which aligned with our results.
In the present study, the period of sample collection included just one day in December. Additionally, there were long stop periods in January and February compared to March. Subsequently, positive samples increased in the spring season. hRV, hPIV3, and hAdV peaked in March, whereas hRSV-A and hEV peaked in January. In Saudi Arabia, hRSV increased in January (5). In Saudi Arabia also, the circulation of respiratory viruses peaked in December (20%), followed by January (14%), March (13%), and February (9%) (24).
All participants were outpatients, and respiratory infections ranged from mild to moderate. Rhinorrhea and cough were the most frequent in positive samples, particularly with hRV. It is known that hRV is abundant in the nose (37), so all hRV patients had rhinorrhea. Likewise, cough, fever, and rhinorrhea were mainly associated with hRV in Peru (32). In China, cough and fever were the most common symptoms in patients infected with hPIV3 (31) and hAdV (30). In our study, hRSV-A and hEV caused more fever than other viruses. Dyspnea and tachypnea were primarily associated with hPIV3. Likewise, a previous study found that dyspnea was more common in hPIVs-positive than in hPIVs-negative (31).
The present study is considered good data for the epidemiology of respiratory viruses among children in the Al-Ahsa region, Saudi Arabia. This study has permitted the detection of eight viruses by multiplex PCR/RT-PCR. The used technique saved time and effort and gave good results. High-positive samples with high co-infection were recorded. The only limitation of this study included no infants among patient.
We thank all the parents of the pediatric patients who participated in this study. In addition, all thanks and appreciation to Nurse. Fatimah Al-Arik and Nurse. Abeer Nour Al-Din from the polyclinics of KFU for their assistance in the collection of samples.
Ethics approval
The ethical approvals were obtained from the ethics committee of the Deanship of Scientific Research of KFU (Agreement KFU-REC/2020-10-13) for samples collected from the KFU polyclinic patients.
Conflicts of Interest
The authors declared no conflict of interest.
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT Nb. 4268].
Received: 2023/09/19 | Accepted: 2023/12/12 | ePublished: 2024/01/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |