BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2131-en.html


 , Firas Rahi Alhachami2
, Firas Rahi Alhachami2 
 , Mohammed Ameer Abdullah3
, Mohammed Ameer Abdullah3 

 , Ahmed S. Abed4
, Ahmed S. Abed4 

 , Mohammed Hassib Ali5
, Mohammed Hassib Ali5 
 , Raji Mosen Al-Yasiri5
, Raji Mosen Al-Yasiri5 
 , Raed Shakir Shnain6
, Raed Shakir Shnain6 
 , Qais R. Lahhob7
, Qais R. Lahhob7 


2- Department of Biology, College of Education for Pure Science, Wasit University, Wasit, Iraq
3- Department of Microbiology, Ninevah College of Medicine, Ninevah University, Mosul, Iraq
4- Jabir Ibn Hayyan University of Medical and Pharmaceutical Sciences, Najaf, Iraq
5- Dhi-Qar Health Directorate, Iraqi Ministry of Health, Nasiriya, Iraq
6- Alshaheed fayrooz hospital, Wasit Health Directorate, Iraqi Ministry of Health, Wasit, Iraq
7- College of Pharmacy, National University of Science and Technology, Dhi Qar, Iraq ,
Staphylococcus haemolyticus is a commonly encountered pathogen responsible for staphylococcal infections and is the predominant and clinically significant species among Coagulase-negative staphylococci (CoNS) (1-4). The rapid adaptation and development of antibiotic resistance, including methicillin resistance, and its ability to persist in healthcare settings underscore the significance of multi-resistance in assessing the risk posed by this pathogen (5-7). Methicillin-resistant S. haemolyticus isolates have been associated with sepsis and increased patient morbidity and mortality rates, thus emphasizing the clinical impact of antibiotic resistance (8-10). Moreover, S. haemolyticus demonstrates remarkable versatility and adaptability within the hospital environment and medical devices, making it a key contributor to nosocomial infections (11, 12).
Staphylococcus haemolyticus is frequently isolated from human urine and wound swab cultures, ranking second only to Staphylococcus epidermidis among CoNS species. It plays a pivotal role in healthcare-associated opportunistic infections, particularly those related to implanted medical devices. Among CoNS, S. haemolyticus exhibits the highest level of antibiotic resistance, with common heteroresistance to glycopeptides (13-15). Its capacity to develop biofilms and colonize hospital environments further contributes to its persistence and pathogenicity, leading to infections such as endocarditis, urinary tract infections, septicemia, and peritonitis. The genome of S. haemolyticus encodes enzymes involved in forming the polygamma-glutamate capsule, which provides defense against cationic antimicrobial peptides (1, 16, 17).
Staphylococcal hemolysins, classified into four toxin types (alpha, beta, gamma, and delta), play a significant role in the virulence of S. haemolyticus. The hla gene, encoding a pore-forming cytotoxin (PFT), targets various human cell types and acts as a detergent on erythrocytes. Another notable feature of S. haemolyticus is its capacity to form biofilms. Bacterial exopolysaccharides generated during biofilm formation may restrict growth and promote the formation of structured biofilms (18-20). Additionally, fibronectin, a glycoprotein found in host tissues, facilitates adhesion between Staphylococcus aureus cells and host cells, playing a crucial role in eukaryotic cell adhesion (21, 22).
This study aimed to investigate antibiotic sensitivity, biofilm formation capabilities, and the presence of virulence-associated genes (hla, hlb, fnbA, and fnbB) in S. haemolyticus isolates. Our study uniquely focuses on pregnant women with urinary tract infections caused by S. haemolyticus. Employing a comprehensive approach, including molecular characterization, antibiotic resistance profiling, and assessment of virulence factors and biofilm formation, we sought to provide accurate insights into the pathogenicity and antimicrobial resistance mechanisms of S. haemolyticus. By addressing these research gaps, our study contributes valuable knowledge to the field, enabling the development of tailored prevention and treatment strategies for this population.
This study was conducted in Al-Rifai General Hospital from October 2021 to December 2022 to isolate S. haemolyticus from clinical samples. The study obtained ethical approval from the Iraqi Ministry of Health. A total of 260 urine swab samples were cultured on CLED agar (Cystine Lactose Electrolyte-Deficient agar) obtained from (Oxoid, Ltd., Basingstoke, Hampshire, England). The suspected S. haemolyticus isolates were subjected to mannitol fermentation, catalase, and coagulase tests followed by API staph identification. Further confirmation of identification was conducted using the VITEK-2 system. Antibiotic sensitivity testing was also conducted using the VITEK-2 system with relevant cards to confirm the susceptibility patterns of isolated S. haemolyticus strains against different antibiotics. Semi-quantitative determination of biofilm formation was carried out following the microtiter plate assay method.
The biofilm production assay was performed using the microtiter plate method. Overnight cultures of S. haemolyticus isolates were diluted in tryptic soy broth supplemented with glucose and added to 96-well microtiter plates. After 24 hours of incubation at 37°C, non-adherent cells were removed by washing, and the biofilms were fixed with methanol and stained with crystal violet. OD570 values were measured after solubilizing the stain with acetic acid. Biofilm production was categorized as negative (OD570 ≤ 0.1), weak (0.1 < OD570 ≤ 0.5), or strong (OD570 > 0.5) based on the optical density values.
The DNA was extracted from S. haemolyticus isolates using the PrestoTM Mini gDNA Bacteria Kit Quick Protocol (Geneaid), and PCR amplification was performed to detect the presence of virulence-associated genes (hla, hlb, fnbA, and fnbB) in the S. haemolyticus isolates. The primer sequences used for the amplification of each gene as well as the expected PCR product size are included in Table 1. Moreover, Table 2 provides details of the PCR cycling parameters and conditions used to detect these genes.
Table 1. The primer sequences and expected PCR product size of each gene
| The gene | The primer sequences | Expected PCR product size |
| hla | Forward primer: 5'-ATGAAAAAGCCTGAAAGAA-3' Reverse primer: 5'-TTATTTTACATCCATACTTATG-3' |
800 base pairs |
| hlb | Forward primer: 5'-TCTAATGATTTGACTAAAGT-3' Reverse primer: 5'-TTACTTATTTTATGTTTGGT-3' |
900 base pairs |
| fnbA | Forward primer: 5'-ATGAAAAAGCCTGAAAGAA-3' Reverse primer: 5'-TTATTTTACATCCATACTTATG-3' |
1000 base pairs |
| fnbB | Forward primer: 5'-TCTAATGATTTGACTAAAGT-3' Reverse primer: 5'-TTACTTATTTTATGTTTGGT-3' |
1200 base pairs |
Table 2. PCR Cycling Parameters and Conditions
| PCR steps | Temp. | Time | Repeat |
| Initial Denaturation | 95 °C | 5min | 1 cycle |
| Denaturation | 95 °C | 30sec. | 1 cycle |
| Annealing | 55 °C a, b | 30sec. | 38 cycles |
| Extension | 72 °C | 1 min. | 1 cycle |
| Final extension | 72 °C | 5min. | 1 cycle |
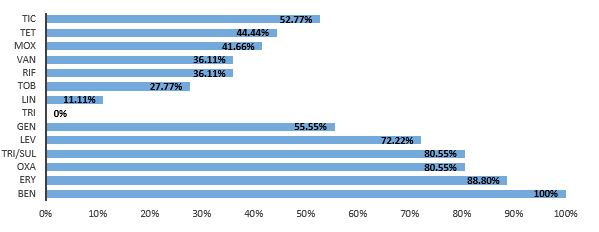
In this study, a total of 260 clinical samples were tested, from which 36 S. haemolyticus isolates were identified. The antibiotic resistance profile of these isolates was assessed as illustrated in Figure 1, revealing a high level of resistance to multiple antibiotics. The isolates demonstrated complete resistance to Benzylpenicillin (100%) and high resistance rates to Erythromycin (88.8%), Oxacillin (80.55%), Trimethoprim/ sulfamethoxazole (80.55%), Levofloxacin (72.22%), and Gentamicin (55.55%). On the other hand, lower resistance rates were observed for Tigecycline (0%), Linezolid (11.11%), Tobramycin (27.77%), Rifampin (36.11%), Vancomycin (36.11%), Moxifloxacin (41.66%), Tetracycline (44.44%), and Ticoplanin (52.77%). Statistical evaluation of antibiotic resistance demonstrated a significant difference (p=0.0001) between resistant and sensitive isolates for all tested antibiotics, except for Moxifloxacin, where no significant difference (p > 0.05) was observed.
Figure 1. Antibiotic Resistance Profile of S. haemolyticus Isolates, where TIC= Ticoplanin, TET= Tetracycline, MOX= Moxifloxacin, VAN= Vancomycin, RIF= Rifampin, TOB= Tobramycin, LIN= Linezolid, TRI= Tigecycline, GEN= Gentamicin, LEV= Levofloxacin, TRI/SUL= Trimethoprim/sulfamethoxazole, OXA= Oxacillin, ERY= Erythromycin, and BEN= Benzylpencillin
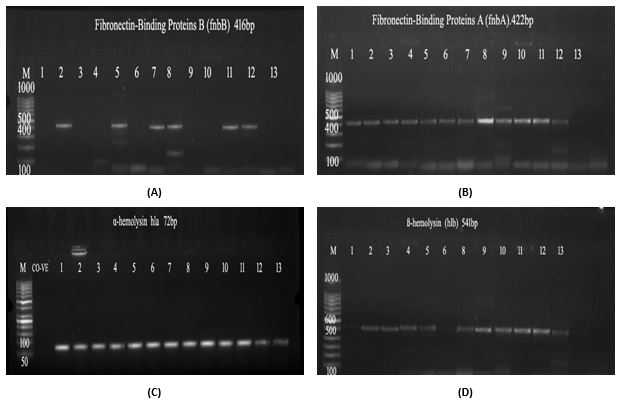
Furthermore, this study investigates the biofilm formation capabilities of S. haemolyticus and explore the presence of virulence-associated genes. The analysis revealed a PIA-independent biofilm formation in S. haemolyticus, distinguishing it from ica-negative isolates. Among the 36 isolates tested, 25 (69.4%) were found to be negative for biofilm formation, while 11 (30.6%) showed weak biofilm formation. No isolates exhibited strong biofilm formation. Notably, all 36 isolates demonstrated the presence of the α-hemolysin gene (hla), while the β-hemolysin gene (hlb) was detected in 28 out of 36 isolates, representing 77.7% of the samples, as determined through PCR analysis. Additionally, the prevalence of Fibronectin A (fnbA) and B (fnbB) genes was observed in 88.8% and 38.8% of the isolates, respectively, as shown in Table 3. To further corroborate these findings, gel electrophoresis was employed, visually confirming the amplified presence of these virulence-associated genes (Figure 2: (a) fnbB gene, (b) fnbA gene, (c) hla gene, and (d) hlb gene).
Table 3. Presence and Absence of Genes in the Samples
| Gene | No. of samples | No. of positive samples | No of negative sample | Percentage of sample positive |
| α-Hemolysin | 36 | 36 | 0 | 100 % |
| ß-Hemolysin | 36 | 28 | 8 | 77.7 % |
| Fibronectin-Binding Proteins A | 36 | 32 | 4 | 88.8 % |
| Fibronectin-Binding Proteins B | 36 | 14 | 22 | 38.8 % |
| Chi-Square: χ2 (P-value) |
--- | --- | --- | 10.372 ** (0.00982) |
** (P≤0.01)-Highly Significant.
Figure 2. Gel electrophoresis of (a) fnbB gene, (b) fnbA gene, (c) hla gene, and (d) hlb gene in S. haemolyticus
Moreover, Table 4 presents the types of drug resistance observed in the S. haemolyticus isolates. Multiple drug resistance (MDR) was highly prevalent, accounting for 91.7% of the isolates. Three isolates (8.3%) exhibited extensive drug resistance (XDR). No isolates showed pan drug resistance (PDR). To determine these resistance patterns, we assessed the susceptibility of S. haemolyticus isolates against a panel of different antibiotics using the VITEK-2 system. The isolates that showed resistance to two or more classes of antibiotics were classified as MDR, while those resistant to nearly all but two or fewer antimicrobial categories were classified as XDR. Isolates that displayed resistance to all agents in all antimicrobial categories were classified as PDR.
Table 4. Types of Drug Resistance in S. haemolyticus Isolates
| Type of resistance | No. of isolates | Percentage of sample (%) |
| DR | 0 | 0 |
| MDR | 33 | 91.7 |
| XDR | 3 | 8.3 |
| PDR | 0 | 0 |
| Total | 36 | 100 |
DR=drug resistance, MDR=Multiple drug resistance, XRD=Extensive Drug Resistance, PDR= Pan drug resistance
The current study revealed a high level of antibiotic resistance among S. haemolyticus isolates, with multiple antibiotics showing high resistance rates. Complete resistance was observed for Benzylpenicillin, followed by high resistance rates for Erythromycin, Oxacillin, Trimethoprim/ sulfamethoxazole, Levofloxacin, and Gentamicin. These findings are consistent with previous studies (23, 24) and suggest that S. haemolyticus has become increasingly resistant to multiple antibiotics.
Sing et al. reported a lower resistance rate to Erythromycin (17%) in their S. haemolyticus isolates (25). On the other hand, Tigecycline, Linezolid, Tobramycin, Rifampin, Vancomycin, Moxifloxacin, Tetracycline, and Ticoplanin showed lower resistance rates in this study compared to previous reports. However, an Iraqi research study reported a Linezolid resistance rate of 4.35% among S. haemolyticus isolates (26). The rising resistance to Linezolid raises concerns, as it is frequently used for gram-positive cocci infections (23). Resistance to Linezolid may be caused by mutations in the cfr or 23S rRNA gene (24). Whole-genome sequencing revealed the remarkable genomic flexibility and phenotypic acquisition of antibiotic resistance in S. haemolyticus (25).
In our study, Tigecycline, a glycylcycline antibiotic, showed complete sensitivity, consistent with previous findings (27). Additionally, combining Tigecycline with Rifampin has been found to have a strong impact on biofilm-forming S. haemolyticus (28). This suggests that Tigecycline, alone or in combination with other antibiotics, could be a potential treatment option for S. haemolyticus infections. Statistical analysis of antibiotic resistance revealed a significant difference (p=0.0001) between resistant and sensitive isolates for all tested antibiotics, except for Moxifloxacin, where no significant difference (p > 0.05) was observed. This indicates that the observed resistance patterns are not due to chance.
Biofilm formation in S. haemolyticus appears to be Polysaccharide Intercellular Adhesin (PIA)-independent, as biofilm formation has been reported in ica-negative isolates. Similar research conducted by (29) has shown that S. haemolyticus and other staphylococci isolated from community contexts lacked the icaAD and bap genes (30). These findings suggest alternative mechanisms may be responsible for biofilm formation in S. haemolyticus strains.
The presence of the α-hemolysin gene (hla) was detected in all 36 S. haemolyticus isolates, while the β-hemolysin gene (hlb) was present in 28 out of 36 isolates. This indicates a high prevalence of these hemolysin genes in S. haemolyticus strains, which may contribute to their virulence. The presence of Fibronectin A (fnbA) and B (fnbB) genes in S. haemolyticus isolates suggests that fibronectin-binding proteins may play a role in bacterial attachment to surfaces and biofilm formation.
The high prevalence of multiple drug resistance (MDR) among S. haemolyticus isolates is consistent with previous studies (29, 31). The widespread use of antimicrobial agents, acquisition of mobile genetic elements, genomic rearrangements, and mutations are factors that contribute to the emergence of antibiotic resistance in S. haemolyticus. The resistance to Vancomycin and Linezolid is particularly concerning, which highlights the clinical impact of S. haemolyticus, especially as methicillin-resistant strains have become common nosocomial pathogens (32). It is imperative to address the challenge of antibiotic resistance in S. haemolyticus for effective infection control in healthcare settings (30).
In conclusion, this study sheds light on the pathogenicity and antimicrobial resistance of Staphylococcus haemolyticus. The isolates exhibited high resistance rates to various antibiotics, with complete resistance to Benzylpenicillin and concerning trends in Linezolid resistance. The presence of virulence-associated genes, including hla and fibronectin-binding proteins fnbA and fnbB, underscores their potential role in the pathogenicity of S. haemolyticus. Biofilm formation appears to be PIA-independent. The prevalence of multiple drug resistance (MDR) highlights the urgent need for effective infection control in healthcare settings. This research provides valuable data for tailored prevention and treatment strategies, contributing to the understanding and managing S. haemolyticus infections.
'Not applicable.’
'Not applicable.’
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2023/06/10 | Accepted: 2023/07/20 | ePublished: 2023/07/26
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |