BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2110-en.html


 , Hadi Shafiee2
, Hadi Shafiee2 

 , Mohamadreza Bayatiani3
, Mohamadreza Bayatiani3 

 , Mohammadbagher Rokni4
, Mohammadbagher Rokni4 

 , Reza Ghasemikhah5
, Reza Ghasemikhah5 


2- Department of Chemistry, Faculty of Science, Arak Branch, Islamic Azad University, Arak, Iran
3- Department of Radiotherapy and Medical Physics, Faculty of Paramedicine, Arak University of Medical Sciences, Arak, Iran
4- Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
5- Department of Parasitology and Mycology, School of Medicine, Arak University of Medical Sciences, Arak, Iran ,
Hydatidosis is a zoonotic chronic parasitic disease with a worldwide spread caused by Echinococcus granulosus. The parasite matures in the intestines of dogs and wild carnivores such as foxes and wolves as definitive hosts (1, 2). The larval stage of the parasite (hydatid cyst) occurs after eating the parasite's eggs in ruminants as intermediate hosts and humans as biological dead-ends (2).
Hydatid cysts can occur in any organ, but the liver and lungs are the two most common organs for disease (3). The disease is more common in Middle Eastern countries such as Iran and Turkey, Mediterranean countries, South America, North Africa, New Zealand, and Australia (2, 4). The World Health Organization estimates that more than one million people worldwide are infected with the disease each year, resulting in considerable yearly deaths, which were 19,300 deaths worldwide in 2015 (4).
There are currently three main treatments for hydatid cyst disease: surgery, percutaneous aspiration (PAIR), and medication. Although surgery is the most effective treatment method, it has many side effects and is often considered dangerous. In addition, the use of sporicidal drugs before and during surgery to prevent the spread of infection is associated with several local and systemic side effects. The drugs are used for adjuvant therapy, which is not adequate alone (5, 6). Recently, non-invasive therapies such as the induction of low-voltage electricity and electromagnetic waves have been studied, which have had limited scolicidal effects (7).
Focused ultrasound is a new non-invasive method that can concentrate high-energy ultrasonic waves in a specific location, leading to cell death and tissue necrosis without damaging other areas. In recent decades, numerous studies on the application of this method in various fields have been conducted, and successful steps have been made by designing ultrasound reactors in the fields of food decontamination, disinfection of surgical instruments, different types of implants, and water purification (8, 9). Moreover, with the invention of high-intensity focused ultrasound (HIFU) converters which use ultrasonic waves and a non-invasive method for tissue destruction in different cancers, several trials have been done, and many of these methods have been approved by the Food & Drug Administration (10).
No ideal method has been found yet to fully treat hydatid cysts with few complications. Therefore, due to the pathophysiological importance of this disease in the body, we aimed to investigate the synergistic effect of focused ultrasonic waves and albendazole sulfoxide on hydatid cyst protoscolices to provide a new therapeutic solution in vitro, aiming to use it as an affordable and less complicated solution for the treatment of patients in the future.
Preparation of Hydatid Cyst Sample
After obtaining the ethics number (IR.TUMS.MEDICINE.REC.1398.721) from the Ethics Committee of Tehran University of Medical Sciences and coordinating with the General Veterinary Office of Markazi Province, naturally infected sheep livers with E. granulosus cysts were collected from the Arak industrial slaughterhouse.
Extraction of Protoscolices
Hydatid cyst-infected livers were immediately transferred to the Arak University of Medical Sciences parasitology laboratory. The fluid in non-infectious, transparent, young, and non-calcified cysts was aspirated with a sterile syringe and poured into sterile Falcon tubes under the hood and sterile condition.
Protoscolices Viability Determination
The viability of protoscoleces was evaluated under a light microscope with lenses 10 and 40 after adding 0.1% eosin. In the samples placed under the microscope, there were approximately 500 to 600 protoscolices, and in each tube, there were around 200,000 to 300,000 protoscolices. The dead protoscolices were red, and the alive protoscolices were green. This part of the process was repeated twice.
Then the samples were divided into four groups:
1. The group that was affected by albendazole sulfoxide alone
2. The group that underwent ultrasound alone
3. The group that was affected by albendazole sulfoxide and ultrasound
4. Control Group
Albendazole Sulfoxide Group
Albendazole sulfoxide was purchased purely from Sigma company, Germany with the CAS registry number of 540-29-12-8. Since albendazole sulfoxide does not dissolve in water, Dimethyl sulfoxide (DMSO) was used as the solvent. According to the studies conducted, the maximum dose of DMSO without a lethal impact on cells is 0.001 g/mL, and this dose was also included in our study for the solubility of albendazole sulfoxide without protoscolex death (11). At first, 10 mg of albendazole sulfoxide was added to 400 μg of DMSO and mixed with the magnetic stirrer. The result was a milky solution containing 2500 μg/ml albendazole sulfoxide. Then, 10 μL of the resulting solution was dissolved into 9990 μL of water to obtain 25 μg/mL albendazole sulfoxide. This also decreased DMSO concentration to 0.001 μg/mL. Albendazole sulfoxide solutions of 25 μg/mL and 12.5 μg/mL were added to protoscolices in the first group of samples. One milliliter of the aforementioned solutions was added to microtubes containing 3000 to 5000 protoscolices. After 24 hours of incubation at 37°C, 10 μL of protoscolices were extracted and placed on a slide with a drop of 0.1% Eosin, and the protoscolices' mortality rate was reported. The experiment was repeated twice.
Ultrasound Group
The ultrasound device was manufactured in Switzerland with a frequency range of 18 to 25 kHz and a maximum power of 100 kW. The waves' intensity was 5-25w/cm2, and the applied frequency was 19.298 kHz.
In the ultrasound-affected group which is the second group of samples, 12 samples were separately exposed to intensities of 5, 15, and 25 w/cm2 in the time intervals of 5, 9, 12, and 18 seconds. Finally, the mortality rate of protoscolices was evaluated by 0.1% Eosin under the light microscope. Two more separate times the experiment was carried out.
Combination Group
For the third group of samples, at first, tubes containing hydatid cyst protoscolices were exposed to albendazole sulfoxide with 25 μg/ml and 12.5 μg/ml concentrations. After 24 hours, similar to the previous group, the samples were exposed to the ultrasound intensities of 5, 15, and 25 w/cm2 in time intervals of 5, 9, 12, and 18 seconds. Then, the mortality rate of protoscolices was evaluated. Each experiment was repeated twice.
Control Group
A control group, the fourth and last group of samples, was also considered in parallel with each experiment and in similar conditions to determine the effect of confounding factors on protoscolex mortality, such as ambient temperature. Each test had one control, which, based on the three testing times in each group, is equivalent to three controls for each of the groups. The average protoscolex mortality rate in these three control samples was calculated. Then, the final mean of the control samples’ protoscolex mortality rate from all three groups was calculated.
Data Analysis
Using SPSS software version 23 (version 23, SPSS Inc, Chicago, IL) and descriptive statistical methods, the mean and standard deviation of the variables were evaluated. Two-way analysis of variance, the Kruskal-Wallis test, and the Mann-Whitney test were utilized to analyze the data.
The Effect of Focused Ultrasound Effect
The protoscolex mortality rates in different durations and intensities of ultrasonic waves have been reported in Table 1. A two-way analysis of variance was used to compare the interaction between the two variables of exposure period and intensity on the percentage of protoscolices death. The hypothesis of homogeneity of variance was evaluated using the Leven test and the results showed homogeneity of variance. The interaction between the two variables of exposure period and intensity was significant (F=530.340, P=0.001). On the other hand, the main effect of each of the two variables of the exposure period (F=64.467, P=0.001) and intensity (F =179.932, P=0.001) was also significant. Figure 1 illustrates the significance of the interaction between exposure period and intensity.
The Effect of Albendazole Sulfoxide
According to Table 1, the mortality rate of protoscolices exposed to 12.5 μg and 25 μg of albendazole sulfoxide was 49.1% and 57.8%, respectively. Based on the results of the Kruskal-Wallis test, no significant difference was observed between the percentage of protoscolices death in albendazole sulfoxide groups of 12.5 μg and 25 μg and the control group (P=0.051). In addition, in binary comparisons between albendazole sulfoxide 12.5 μg and control group (P=0.1) and albendazole sulfoxide 25 μg and control group (P=0.1), and albendazole sulfoxide 12.5 and albendazole sulfoxide 25 μg group (P=0.4) no statistical significance was observed.
Using the Mann-Whitney test, significant results were observed in the comparison of ultrasound group 18 seconds-15 w/cm2 and 18 seconds-25 w/cm2 with albendazole sulfoxide (P=0.037), While other values of ultrasound death percentage with both albendazole compounds did not show significant results (P=0.05).
The Effect of Combination of Focused Ultrasound and Albendazole Sulfoxide
The mortality rate of protoscolices in the group exposed to the combination of albendazole sulfoxide and ultrasound was 100% in all doses of albendazole sulfoxide and all time intervals and intensities of ultrasonic waves (Table 1). Figure 2 depicts a sample of slides taken from protoscolices that were exposed to a combination of ultrasound and albendazole sulfoxide. In comparison between the combination group and albendazole sulfoxide group, statistical significance was observed in all exposure durations and intensities of ultrasound (P=0.037). In addition, except for ultrasound 18 seconds-15 w/cm2 and 18 seconds-25 w/cm2, other comparisons revealed a significant difference (P=0.037) between the ultrasound and combination groups.
Table 1. Mortality rate of protoscolices in different groups
| Group | Number of experiments | Mortality rate | |||
| Minimum | Maximum | Mean | Standard. Deviation | ||
| Control | 3 | 2.70 | 7.30 | 5.1333 | 2.31157 |
| Albendazole 12.5 μg | 3 | 41.70 | 55.40 | 49.1000 | 6.91592 |
| Albendazole 25 μg | 3 | 47.50 | 66.60 | 57.8000 | 9.63795 |
| Ultrasound 5w/cm2_5s | 3 | 74.00 | 77.50 | 75.5000 | 1.80278 |
| Ultrasound 5w/cm2_9s |
3 | 90.50 | 91.40 | 90.8667 | 0.47258 |
| Ultrasound 5w/cm2_12s |
3 | 95.70 | 96.60 | 96.1333 | 0.45092 |
| Ultrasound 5w/cm2_18s |
3 | 99.20 | 99.70 | 99.4333 | 0.25166 |
| Ultrasound 15w/cm2 _5s |
3 | 87.60 | 88.30 | 87.9667 | 0.35119 |
| Ultrasound 15w/cm2_9s |
3 | 94.90 | 95.30 | 95.1000 | 0.20000 |
| Ultrasound 15w/cm2_12s |
3 | 96.00 | 99.20 | 97.9000 | 1.68226 |
| Ultrasound 15w/cm2_18s |
3 | 100.00 | 100.00 | 100.0000 | 0.00000 |
| Ultrasound 25w/cm2_5s |
3 | 91.80 | 93.30 | 92.4333 | 0.77675 |
| Ultrasound 25 w/cm2_9s |
3 | 95.10 | 96.40 | 95.6667 | 0.66583 |
| Ultrasound 25w/cm2_12s |
3 | 97.50 | 98.80 | 98.2333 | 0.66583 |
| Ultrasound 25w/cm2_18s |
3 | 100.00 | 100.00 | 100.0000 | 0.00000 |
| Combinationa |
3 for each testb | 100.00 | 100.00 | 100.0000 | 0.00000 |
aAll intensities and durations with both doses of albendazole sulfoxide showed a similar result
bAll the mentioned intensities and time intervals related to ultrasound were applied in combination with doses of 25 μg and 50 μg albendazole sulfoxide. Each of the tests was performed three times
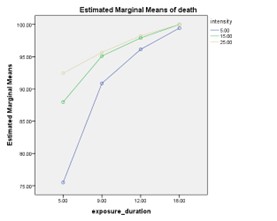 |
Figure 1. The mortality rate of protoscolices at different time intervals of exposure and intensities of focused ultrasound |
|
|
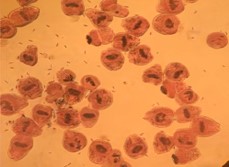
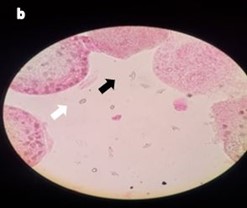
Figure 2. 100% mortality of the protoscience after exposure to albendazole sulfoxide 12.5 µg/mL and ultrasound for 5 seconds at 15 w/cm2 and staining with eosin 0.1% (a: 10x magnification, b: 40x magnification). The protoscolices were fragmented, hooks (black arrow) and calcareous corpuscles (white arrow) were released and scattered around (b)
The present study demonstrated that ultrasonic waves with the applied intensity and duration can cause damage to protoscolices and ultimately result in their death. The development of noninvasive methods for killing protoscolices has shown promise in reducing the invasiveness and potential complications associated with surgery. Radiotherapy for hydatid cysts, although not commonly employed, may potentially contribute to cyst shrinkage and sterilization of residual cysts, but its efficacy is limited, and it carries the risk of radiation toxicity and the development of secondary tumors (12, 13). Thermal ablation techniques, such as radiofrequency or laser ablation, have the potential to effectively destroy hydatid cysts and sterilize the cyst contents, but their applicability may be influenced by cyst location and size, and there are risks of cyst rupture, inflammatory response, and limited clinical evidence (14). Therefore, it is still necessary to find a new noninvasive method to minimize complications, and according to this study, the use of high-intensity ultrasound waves in conjunction with albendazole administration may be a suitable choice.
In our study, Changes in protoscolices' morphology and mortality rate after exposure to high-intensity ultrasound were shown to be dose and time-dependent, with protoscolex mortality reaching 100 percent at 15 w/cm2 and 18s. In this context, the results of this study are consistent with the results of the Zou et al. study. In their study, they observed the destructive effect of HIFU on protoscolices and its growth-inhibiting effect on hydatid cysts. These effects were directly proportional to the intensity of the waves and their application duration, with protoscolices mortality reaching its peak at 200 w and 40 s, or 250 w and 20 s, which was greater than our study's findings (15). Similarly, the study by J. Liu et al. demonstrated that the damage to the cyst wall and protoscolices was directly related to the intensity and duration of ultrasonic waves (16).
The biological effects of ultrasonic waves on the structure and function of living systems vary based on the intensity of the waves. At low intensities, focused ultrasonic wave radiation mildly raises the temperature and causes mechanical stimulation, weakening the tight connections between cells and increasing drug penetration into target tissues (17). At higher intensities, the cavitation effect occurs, which means that microscopic gas bubbles form in the ultrasound field, increasing the temperature of the surrounding environment (18). Above the specific pressure threshold, the bubbles burst and cause damage to the local tissue at the focal point of the waves. At very high intensities, the absorbed energy is quickly converted to heat due to pressure fluctuations at the focus area, causing coagulation necrosis (19).
Moreover, Jing Zhang's study has shown that due to the conductivity of the liquid in which protoscolices are located, ultrasound intensity of about 100W and a frequency of 0.8 MHz cannot increase the temperature to the point causing necrosis (20). our experiment has a similar argument, even though the ultrasound intensity in our study is much lower than that in his study. Altogether, factors such as acoustic energy transfer, cavitation phenomenon, and subsequent increase in spot temperature at the bubble site justify changes in protoscolices morphology and their death, as shown by vital staining in the present study.
It is important to note that ultrasound waves have been utilized in the non-invasive treatment of certain types of malignancies with minimal side effects. In a study involving 1742 patients with prostate cancer, Dosanjh et al. found that HIFU treatment was associated with a similar rate of complications as conventional therapies. (21). Also, in Feril et al. study on the effect of HIFU on Breast cancer, HIFU had good therapeutic effects with low complications making it an attractive alternative for some individuals (22).
Although the non-invasive approach of transferring energy by acoustic waves to biological targets such as solid tumors or protoscolices can be interesting, the fluid in the cyst prevents microorganisms from absorbing energy. It may take greater acoustic energy intensities to destroy them. This issue raises concerns over the healthy tissue surrounding the target or the tissue in the radiation's path. In certain experiments, such as the one by Lui et al., ultrasonic absorbents and ultrasound contrast agents have been utilized to minimize the required ultrasonic wave intensity (23). In our study, albendazole sulfoxide was employed to induce synergistic effects, and samples were placed at 37 degrees to simulate body temperature. The combination of focused ultrasound and albendazole sulfoxide significantly increased the mortality rate of protoscolices, and even at lower intensities of ultrasonic waves, 100% protoscolices mortality was observed.
One of our study's limitations was that the non-lethal concentration of DMSO was based on previous studies on cells, and due to the lack of protoscolices, it was impossible to determine the certainty of its non-lethal effect on protoscolices. In addition, the concentration of albendazole sulfoxide considered in the study is high, and this concentration is rarely observed in the blood. Therefore, these experiments should be repeated with lower doses of albendazole sulfoxide. It is suggested that future research assess the in vivo effects of consuming albendazole followed by exposure to focused ultrasonic waves on hydatid cysts of the liver and lung.
The current study demonstrated that ultrasonic waves alone can effectively destroy hydatid cyst protoscolices. Also, the synergistic effects of ultrasonic waves and albendazole sulfoxide make it possible to achieve complete death of protoscolices with lower doses of albendazole sulfoxide and lower intensity and duration of ultrasonic irradiation. This is important because it results in less damage to healthy tissue around the target or in the path of radiation. On the other hand, it reduces the need for high doses of albendazole, hence decreasing the likelihood of drug adverse effects. Importantly, with this low-risk procedure, it is possible to eradicate hydatid cysts in various parts of the body, and it can serve as an alternative to surgery. To acquire more definitive results, however, albendazole and focused ultrasonic waves must be applied in clinical settings.
We are sincerely grateful to the General Directorate of Veterinary Medicine and Slaughterhouse of Arak for their assistance in progressing our research .
The ethical approvals were obtained from the Ethics Committee of the Tehran University of Medical Sciences with the ethics number of IR.TUMS.MEDICINE.REC.1398.721.
Conflicts of Interest
The authors disclose no conflicts of interest.
This research did not receive any funding.
Received: 2023/08/22 | Accepted: 2024/01/4 | ePublished: 2024/01/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |