BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1903-en.html


 , Zahra Akbari Jonoush2
, Zahra Akbari Jonoush2 

 , Mehri Ghafourian2
, Mehri Ghafourian2 

 , Seyed Esmaeil Khoshnam3
, Seyed Esmaeil Khoshnam3 

 , Fereshteh Nezhad Dehbashi4
, Fereshteh Nezhad Dehbashi4 

 , Maryam Farzaneh5
, Maryam Farzaneh5 


2- Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
3- Persian Gulf Physiology Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
4- Cellular and Molecular Research Center, Medical Basic Science Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
5- Cellular and Molecular Research Center, Medical Basic Science Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran ,
On December 31, 2019, some patients who developed pneumonia of unknown cause were reported to the World Health Organization (WHO) by the China Health Authority (1, 2). Coronavirus disease 2019 (COVID-19) emerged as a global public health threat and spread rapidly around the world (3, 4). It was later renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the Coronavirus Study Group, then a group of virologists in China proposed to rename SARS-CoV-2 as "human coronavirus 2019" (HCoV-19) to distinguish it from SARS-CoV (5, 6). SARS-CoV-2 mainly affects the respiratory system, and common symptoms such as cough, fever and shortness of breath are observed (7). In severe cases, COVID-19 can trigger septic shock and severe acute respiratory distress syndrome (8). Moreover, liver damage caused by COVID-19 is important, which is associated with a transient increase in the amount of transaminases and/or other liver enzymes (9, 10). On average, 15% to 58% of patients with COVID-19 showed elevated levels of liver enzymes (11). Common tests for abnormalities in liver function have shown elevated aminotransferases in patients with SARS-CoV-2, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (12, 13). Many factors cause elevated liver enzymes in patients with SARS-CoV-2, including hepatic ischemia, direct liver injury, cardiac cirrhosis (congestive hepatopathy), drug-induced liver injury (DILI), and muscle atrophy (14, 15). DILI due to excessive use of antivirals, antimalarials, and antimicrobial drugs causes liver function test (LFT) abnormalities that usually resolve with recovery from COVID-19 or discontinuation of the drug (16). Therefore, there is a need for effective and safe treatment methods to decrease the burden of COVID-19 (17). Remdesivir (also GS-5734, RDV) was originally studied for treating Ebola, the epidemic in West Africa, from 2013 to 2016 (18). RDV has been developed as a potential therapeutic drug for COVID-19 and received emergency use authorization from the Food and Drug Administration on May 1, 2020, as it was effective to shortening the recovery time for COVID-19 patients (19, 20). RDV is a monophosphoramidate adenosine analog prodrug with antiviral activity against an array of RNA virus families, which halts RNA replication (21). It is a broad-spectrum antiviral mono-phosphoramidate prodrug that is metabolized in the liver to its active form (RDV triphosphate), which competes with ATP and interferes with the RNA-dependent RNA polymerase (RdRp) activity, thereby disrupting viral RNA replication (22). However, RDV can cause mitochondrial toxicity and inhibits mammalian DNA and RNA polymerases (23). Despite varying efficacy data, current treatment guidelines suggest administering RDV to COVID-19 patients who require supplemental oxygen (24). Currently, initiation of RDV therapy is not recommended in patients with LFT more than the upper limit of normal (ULN), because RDV can increase ALT and AST levels and this warning limits the use of this drug (25). There is currently little evidence regarding RDV hepatotoxicity in vitro. Liver cells are a suitable source for investigating the effects of hepatotoxicity of drugs. However, in vitro culture and maintenance of mammalian hepatocytes’ function as the primary liver cells are challenging (26, 27).
Domestic chicken has recently been reported as a specific evolutionary model connecting mammals and vertebrates (28). Chicken embryo-derived cells exhibit similar responses to mammalian species and can be a suitable source for investigating the effects of drug-induced hepatotoxicity (29-32).
Considering the effective results of RDV in patients with COVID-19 and the impact of COVID-19 on liver function, the in vitro function of RDV in hepatocytes is necessary. Therefore, this study investigated the effects of RDV on the expression and activity of liver enzymes in chicken embryo-derived hepatocytes for the first time.
2.1. Ethics Statement
In this in vitro study, chicken fertilized eggs (Gallus gallus domesticus, White leghorn strain) were obtained from a commercial breeding farm, Ahvaz, Iran. This company raises breeder birds following industry standards to achieve optimal bird performance. This study was conducted in accordance with the guidelines for the care and use of experimental animals established by the Institute for Laboratory Animal Research (ILAR) of Ahvaz Jundishapur University of Medical Sciences (Ethical no. IR.AJUMS.REC.1400.638).
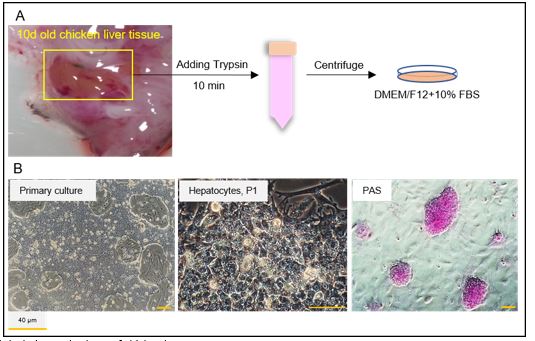
2.2. Isolation of liver tissue from 10d old chicken embryos
Twenty embryonated chicken eggs (stage X) were incubated (37.5 °C, 60-65% humidity) for 10 days (stage HH35). After 10 days, the embryos were separated from the yolk and the extraembryonic tissues. The liver tissue was then aseptically dissected and transferred to a sterile petri dish containing phosphate-buffered saline (PBS). The tissues were cut into small pieces and put into a sterile tube with the Trypsin-EDTA enzyme (0.25% Trypsin and 2.25 mM EDTA). Trypsin is a serine protease that cleaves proteins into peptides. For this purpose, this enzyme was added (10 min) to dissociate the tissue into single cells. Then, the enzyme was inactivated via basic culture medium containing serum (DMEM+10% FBS). After digestion, the suspension was centrifuged at 1200 rpm for 5 minutes and transferred to the gelati (0.1%) coated plates in DMEM/F12+10% FBS medium and incubated at 37 °C and 5% CO2. After two days, the growth and proliferation of hepatocytes were observed using the inverted microscope.
2-3. Periodic acid Schiff (PAS) staining
The cells were fixed with paraformaldehyde (4%, 20 min) and washed with PBS for PAS staining . The Periodic acid solution was used (10 min) and washed (PBS). Then, the Schiff’s solution was added (10 min) and washed (PBS), and the cells were observed with the inverted microscope.
2-4. Addition of RDV
After 3 days (70% confluency), four concentrations of RDV (2.00, 3.00, 4.00, and 5.00 µM) were added to the culture medium, and liver cells were incubated. After 24h and 48h, the cell viability was measured with the Trypan Blue test (33).
2.5. Gene expression analysis
-
Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from tissues and cells by TRIZol reagent (Invitrogen) following the manufacturer’s instructions. RNA quality was assessed by both UV spectrophotometry (Eppendorf) and agarose gel electrophoresis. The purity and quality of RNA can be confirmed based on an absorbance ratio of 260/280 and 260/230. An absorbance above 280 indicates the presence of protein in the sample, while an absorbance above 230 may indicate residual phenol. Ideally, the 260/280 ratio for RNA should be approximately between 1.8-2.2, and the 260/230 ratio should be 2.0-2.2. When evaluating the quality of RNA by gel electrophoresis, the presence of distinct and sharp bands of 28S and 18S ribosomal RNA and the absence of RNA
degraded, indicating integrity and high quality RNA. PrimeScript RT reagent Kit (TaKaRa) was used to synthesize first-strand cDNAs with 1 μg total RNA and stored at -20°C.
-
Quantitative real-time PCR (qPCR)
qPCR was used to detect the expression level of the liver genes on a QuantStudioTM Real-time PCR system (Applied Biosystems). The reaction procedure was as follows: 10 min hold at 95 °C, followed by 40 amplification cycles of 95 °C denaturation for 15 s and 60°C for 1 min. Then, high-resolution melting was performed on the PCR amplicons (95°C for 15 s, 60°C for 1 min, and 95°C for 15 s). After amplification, dissociation curve analysis for each sample were analyzed using Dissociation Curve 1.0 software (Applied Biosystems) to identify and remove possible primer dimer artifacts. Melting curve analysis and direct sequencing of amplicons confirmed the presence of a single PCR product for all primer pairs. Data were analyzed using the 2−ΔΔCT method. Expression values were corrected for the housekeeping gene β-actin.
2-6. The activity of liver enzymes (ALT and AST)
We used a sandwich enzyme-linked immunosorbent assay (ELISA) system to measure the enzyme activity of ALT and AST. Firstly, we diluted Capture Antibody in Coating Buffer and added 100 μL of this Capture Antibody solution to all wells of a 96-well microplate. The plates were incubated overnight at 4°C. After washing the plates four times with phosphate-buffered saline-Tween (PBS-T) 0.05%, plates were blocked with 5% BSA diluted in PBST (200 μL/well) for 2 hours at 37°C. After a washing step, a 100 μL sample solution was added to each well, and the microplates were incubated for 1 hour at 37°C. After horseradish peroxidase-conjugated anti-ALT and AST antibody (100 μL) was added to each well and incubated for 1 hour at 37°C. After washing the plates three times, we added 100 μL of 3,3',5,5'-tetramethylbenzidine (TMB) substrate solution to each well and incubated for 15 minutes at room temperature in the dark. Adding 50 μL/well of 2 M sulfuric acid stopped the enzymatic color reaction . The final measurement was performed at a wavelength of 450 nm, and all samples were processed in duplicate.
2-7. Statistical analysis
Statistical analysis was performed by SPSS version 20 (SPSS Inc., Chicago, IL, USA). All experiments were performed in at least three biological replicates and quantitative results were reported as mean ± standard deviation. If the distribution of quantitative data was normal, the t-test was used for comparison between two groups, and the ANOVA test was used for more comparisons of two groups, and if there was no normal distribution, their non-parametric equivalents were used. P-values less than 0.001 (***) and less than 0.05 (**) between groups were considered significant. SPSS software (version. 21) was used for data analysis.
3-1. Culture of hepatocytes from 10d old chicken embryo
First, we isolated the liver tissue from developmental stage HH36 of chicken eggs according to a previously described method (34). Liver cells were enzymatically digested and plated on culture plates precoated with 0.1% gelatin (Figure 1A). DMEM/F12 with 10% FBS was used from the first day of culture. After 2 days, phase-contrast images revealed the small and mono-layer colonies of chicken hepatocytes. Each hepatocyte had a hexagonal structure with a large nucleus and nucleolus. Besides, glycogen and lipid droplets were accumulated in the hepatocyte cytoplasm. In the PAS staining, the pink color of the PAS-positive cells confirmed the presence of glycogen in hepatocytes (Figure 1B).
Figure 1. Isolation and culture of chicken hepatocytes
A) The liver tissue was separated from 10-day-old chicken embryo (stage HH36), enzymatically digested with trypsin, and placed in a culture plate precoated with gelatin. DMEM/F12 and 10% fetal bovine serum (FBS) were used as a basal culture medium for hepatocytes. B) Phase-contrast images showed hepatocytes colonies with a hexagonal structure, a large nucleus, and a dark nucleolus. The pink colonies showed PAS positive hepatocytes (passage 1).
3-2. Viability of hepatocytes after RDV
After four days, we evaluated the cytotoxicity effects of RDV on hepatocytes. Following staining with the trypan blue, in the presence of 4 and 5 μM RDV, up to 50% of hepatocytes lose their viability after 48 hr (P<0.001) (Figure 2). Therefore, we assessed the effects of 4 μM RDV on hepatocyte enzymes in further experiments.
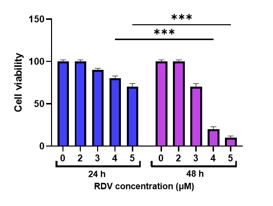
Figure 2. Viability of hepatocytes after RDV
The trypan blue staining was used for the viability test. There was a significant decrease in the hepatocyte’s viability in the presence of 4 and 5 μM RDV compared to other RDV concentration after 48 hr (P<0.001).
**p<0.01 and ***p<0.001 Data were graphed to represent the mean± SEM. Two-way ANOVA were performed to determine the statistical significance of the data.
3-3. The expression of the liver enzyme genes after RDV
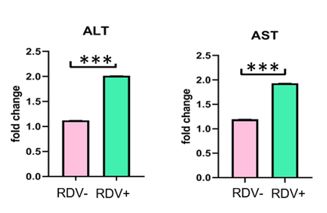
We measured the expression of ALT and AST by qRT-PCR analysis using chicken-specific primers (Table 1). Our data showed that the expression of both ALT and AST were significantly increased after treatment with RDV (P<0.001) (Figure 3).
Figure 3. The expression of the liver enzyme genes after RDV
qRT-PCR analysis was used for ALT and AST expression levels. The expression of both enzymes was significantly increased after treatment with RDV (P<0.001)
***p<0.001 Data graphed to represent the mean± SEM. Unpaired T-test were performed to determine the statistical significance of the data.
Table 1. Primer sequences for chicken related genes
| GENE NAME | SEQUENCE |
| GAPDH-Chik-F | GTCTGGAGAAACCAGCCAAGTA |
| GAPDH-Chik-R | AACCTGGTCCTCTGTGTATCCTA |
| ALT-Chik-F | CTGCATCATCAACCCAGGAAATC |
| ALT-Chik-R | CACATTGTCCTGGTAAACCTCATC |
| AST-Chik-F | ACAATGGCAGATCGGGTCTT |
| AST-Chik-R | TCCACCTGCTTAGGGTTCAA |
| β-Actin-F | ACCTGACAGACTACCTCATG |
| β-Actin-R | ATCGTACTCCTGCTTGCTGA |
Figure 4. The function of liver enzymes after RDV
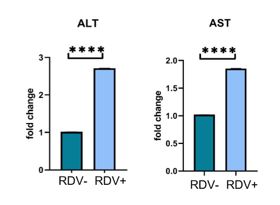
We used the ELISA test to measure ALT and AST functions in hepatocytes. Our data showed that ALT and AST functions were significantly increased in the RDV+ hepatocytes compared to the RDV- cells (P<0.001) (Figure 4).
The function of both ALT and AST was significantly increased after RDV (P<0.001)
***p<0.001 Data graphed to represent the mean± SEM. Unpaired T-test were performed to determine the statistical significance of the data.
Based on previous studies, patients with COVID-19 treated with RDV commonly exhibit elevated liver enzymes (35). Liver disorders can be prognostic factors in the mortality rate of COVID-19 and usually occur in patients with advanced disease (14). Some studies showed that RDV can lead to liver dysfunction or kidney during treatment. Therefore, its administration should proceed with extreme caution (18).
In our study, for the first time, we used chicken embryo-derived hepatocytes as a pre-clinical model to evaluate the side effects of RDV. Chicken embryos are used in many studies due to their accessibility, cost-effectiveness, and rapid growth (28, 36, 37). We found that the mRNA expression levels of hepatocyte-specific enzymes were enhanced after treatment with RDV. Additionally, the activities of ALT and AST increased in the RDV+ groups. This result suggests that RDV can affect the behavior of hepatocytes .
To study liver damage in patients with COVID-19, Zampino et al. reported that RDV caused hepatocytotoxicity. Although the number of patients was small (five patients), a clear increase in ALT and AST was observed after RDV administration (38). Besides, Grein et al. investigated the effect of RDV on the recovery of patients with COVID-19. In one patient, drug injection was stopped due to increased enzyme levels. The results of this group showed that RDV increased the activity of ALT and AST (39). Goldman et al. found that in 2% of patients, the increase in AST/ALT levels can be life-threatening, and suggested reducing or long-term RDV therapy (17). In addition, they showed that the use of RDV significantly reduced the recovery time of patients hospitalized with Covid-19. Indeed, a direct correlation was observed between patients treated with RDV and reversible elevation of ALT/AST without pathological abnormalities in liver or kidney function (17). Interestingly, Carothers et al. displayed that in two patients receiving RDV, the transaminase levels increased between days 3 and 10. This group showed that liver failure improved after continuous infusion of acetylcysteine and discontinuation of RDV (40). These findings suggest that it is important to consider RDV-associated acute liver failure (ALF) against its benefits and acetylcysteine may play a role in its management. These findings are consistent with our results which clearly showed a significant difference in RDV-induced hepatotoxicity between the two groups.
In contrast to our findings, a study by YN Li in China using male C57BL/6 mice showed that the serum levels of ALT and AST in high-fat diet (HFD)-induced non-alcoholic fatty liver disease (NAFLD) mice were significantly reduced after treatment with RDV. This may be due to the difference in the animal model as well as the induction of NAFLD in these mice, which itself increased the level of AST and ALT (41).
A recent placebo-controlled study in hospitalized adults with severe COVID-19, treatment discontinuation occurred more frequently in RDV recipients than placebo recipients due to side effects such as increased levels of aminotransferase or bilirubin (16). Although the FDA and European medicines agency have approved the prescription of RDV for patients with COVID-19, scheduled monitoring of liver function before and during treatment is recommended.
Having chronic liver diseases (such as hepatitis C and/or hepatitis B) with or without elevated transaminase levels can increase the risk of liver damage caused by RDV administration (42, 43). However, studies have shown that the increase in liver enzymes during COVID-19 was not exclusively caused by the administration of RDV. Hypoxia and hypotension associated with pneumonia may also contribute to liver damage or even progression to liver failure in patients with Covid-19 (44). Therefore, it is clear that further investigation of the manifestations, mechanisms, and prognosis of injury damage in patients with COVID-19 is needed for improvement and development of targeted therapies.
We concluded that the expression and function of hepatocyte enzymes were increased following treatment with RDV. In this study, we aimed to raise the awareness of healthcare professionals about RDV-induced liver injury and the management of its treatment in patients with COVID-19. Close monitoring of LFT in COVID-19 patients can provide early detection of liver damage and may reduce the risk of DILI. Further research on chicken hepatocytes is recommended to develop a better understanding of the nature of the risk of hepatotoxicity and drug-drug interactions with RDV in COVID-19 before its use.
Et
Not applicable.
The datasets used and/or analyzed d
Not applicable.
We wish to thank all our colleagues in Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Ethical No. IR.AJUMS.REC.1400.638).
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2023/01/23 | Accepted: 2023/04/29 | ePublished: 2023/06/26
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |