BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1866-en.html
2- Department of Biotechnology, Iranian Research Organization for Science and Technology (IROST), Tehran, Iran
3- The Lister Laboratory of Microbiology, Tehran, Iran
Ongoing outbreak of emerging viral infections had long been a challenge for the public health. In December 2019, the world has witnessed the rise of another viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in the global pandemic of COVID-19 disease (1). This emerging viral infection has occurred while public health has experienced the outbreak of several other viruses including influenza H1N1, Middle East respiratory syndrome (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV), Ebola and Zika (2-7). This novel virus was initially named 2019-nCoV by the Chinese Center for Disease Control and Prevention and then renamed by the Coronaviridae Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV) as SARS-CoV-2 (8). The virus is a close relative of SARS-CoV and belongs to Coronavirinae subfamily and the genus Betacoronavirus (1).
The reference sequence of SARS-CoV-2 has 29903bp length as positive single strand RNA (1). The genome encodes four structural proteins including matrix (M), envelope (E), nucleocapsid (N) proteins and spike (S) glycoprotein and also several nonstructural and accessory proteins (1).
By August 2022, more than 573 million cases and 6.4 million deaths have been confirmed for COVID-19 disease worldwide (https://covid19.who.int/). The considerable ongoing cases and deaths have still made the molecular tests necessary to control the pandemic especially to combat new virus variants. Earlier diagnosis of COVID-19 has been crucial for a proper treatment regimen. A comparison between molecular and serological tests has revealed that nucleic acid-based tests are able to detect SARS-CoV-2 in earlier stages (9). Two main molecular techniques including real-time reverse transcription PCR (rRT-PCR) and isothermal nucleic acid amplification e.g., reverse transcription loop-mediated isothermal amplification (RT-LAMP) have been intensively reported for infectious diseases diagnosis. RT-LAMP is an isothermal field applicable assay with no need for using expensive instruments, highly trained personnel and the capability to detect the viral genome sequence in a very short time while the former one is a standard gold method usually used in clinical labs (10).
LAMP assay is an emerging molecular identification method in the third millennium (11). This technique has opened its place in detecting microorganisms, especially the agents of infectious diseases, including viruses. Instead of two or three primers used in PCR and RT-PCR, four to six primers are used in this technique targeting six to eight regions of the target gene and available results in less than one hour (12). This method includes DNA strands transformation into dumbbell and loop structures in the first stage and then amplification of the loops exponentially in plenty of hairpins of increasing lengths (13).
Several parameters such as primer concentrations are critical to optimize RT-LAMP reaction. Therefore, design of a positive control can facilitate and speed up test setup and ensure that the assay is correctly performed. Since working with clinical specimens may be restricted or not be accessible, the positive control can solve this problem and remove the obstacle to rapid development of RT-LAMP assays where there is no alternative solution to access the genetic materials on time, especially for new emerging variants.
Several ways to introduce positive control into RT-LAMP reaction include gene constructs plasmid DNA, transcribed RNA, armored RNA and inactivated virus (14-17). In this study, two RT-LAMP assays were promptly developed using gene construct design approach at the first step.
Target Gene Selection
The nucleocapsid (N) gene of SARS-CoV-2 is located at nucleotide positions 28274 to 29533 of the NCBI reference genome (NC_045512.2). This gene was selected as target region for our RT-LAMP assays setup because it has higher conserved sequences and high expression during infection, making it useful as target gene (18).
Gene Constructs Design
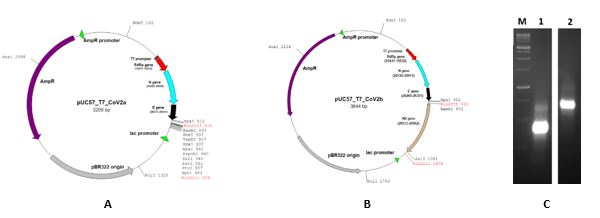
A 499bp DNA fragment was initially designed based on SARS-CoV-2 reference genome (NC_045512) including partial sequences of RNA dependent RNA polymerase (RdRp) gene (15,431–15,530), nucleocapsid (N) gene (28285-28529) and envelope (E) gene (26,270–26,381) flanked by T7 RNA polymerase promoter sequence at its 5’end (Figure 1A). The DNA fragments were artificially synthesized by GenScript, USA, and provided as the gene inserts into the pUC57 plasmid at the EcoRV restriction site in a 4 μg lyophilized powder form named pUC57_T7_CoV2a. The RdRp and E amplicons were the same as described by Corman et al. (19). The N amplicon was selected from the consensus sequence of 35 different SARS-CoV-2 including MT320891, MT447177, MT459928, MT380726, MT380727 strains reported from Iran.
In the next step, a 422bp fragment of N gene (28533-28758) was produced by a nested PCR using nC-N3-F1 and nC-N3-R1 as external primers and then nC-N3-F2 and nC-N3-R2 as internal primers in the first and second PCR reaction (Table 1). For the internal primers and cloning purposes, the BamHI I and SalI sites were introduced into the 5’-end of primer sequences described by Fallah et. al 2003 (20). This gene construct is named pUC57_T7_CoV2b (Figure 1B). The pUC57_T7_CoV2a (p1) lacked N gene amplicon used by US Centers for Disease Control and Prevention (CDC) but pUC57_T7_CoV2b (p2) included this region via second fragment insertion.
Artificial RNA Synthesis
Initially, the gene construct pUC57_T7_CoV2a (p1) in lyophilized powder form was dissolved in 40μL RNase free water and 10μL of it was treated with restriction enzyme HindIII to be linearized for in vitro transcription. The second gene construct, pUC57_T7_CoV2b (p2) is also linearized with StuI. The artificial SARS-CoV-2 RNA was synthesized and purified using the HiScribe™ T7 Quick High Yield RNA Synthesis Kit (New England Biolabs, UK) according to the manufacturer’s instructions. The RNA transcripts concentrations were then determined at 260 nm.
RT-LAMP Primer Specificity Analyses
Initially the SARS-CoV-2 nucleocapsid (N) gene was chosen for RT-LAMP to be utilized as target regions. There were significant mismatches between SARS-CoV-2 (NC_045512.2) and SARS-CoV strains as shown in (Figure 2). The multiple sequence alignment was also conducted for other non- SARS-CoV-2 viruses including Bat SARS-like CoV viruses (MG772934.1, KY417144.1, MG772933.1), Betacoronavirus England 1 (NC_038294.1), Civet SARS CoV (AY304488.1), SARS CoV viruses (NC_004718.3, FJ882957.1, AY395003.1), Middle East Respiratory/MERS CoV (NC_019843.3), Murine hepatitis virus/MHV (NC_001846.1) and Human Coronaviruses HKU1 (NC_006577.2), NL63 (NC_005831.2), OC43 (NC_006213.1), 229E (NC_002645.1). The primers mismatches to non-SARS-CoV-2 viruses were highly significant showing cross reactivity to be unlikely.
RT-LAMP and Nested PCR Primer Design
Sets of primers for two different LAMP reactions targeting 202 and 216 base pairs regions of nucleocapsid (N) gene of reference SARS-CoV-2 genome (NC_045512.2) were designed using PrimerExplorer V5 software developed by Eiken Chemical Co. Ltd. [http://primerexplorer.jp/e/index.html]. A primer set from Baek et al. 2020 was also used in a separate RT-LAMP assay (Table 1). For nested PCR, two primer pairs were designed using Primer3 (Table 1) (21).
Table 1. List of primers used in this study
| Primer | Target genea Nucleocapsid (N) |
Seq (5’-3’) | Target region (bp) | Reaction | Ref |
| nC-N3-F1 | (28530-28962) | AAATTGGCTACTACCGAAGAGC | 480 | 1st PCR | This study |
| nC-N3-R1 | AGCTGGTTCAATCTGTCAAGC | ||||
| nC-N3-F2 | (28533-28758) | CTCGGATCC ACCGAAGAGCTACCAGACG |
422 | 2nd PCR | This study |
| nC-N3-R2 | TCCTGTCGAC TGAGGAAGTTGTAGCACGATTG |
||||
| nC-N1-F3 | (28285-28529) | TGGACCCCAAAATCAGCG | 245 | RT-LAMPb | (22) |
| nC-N1-B3 | AGCCAATTTGGTCATCTGGA | ||||
| nC-N1-FIP | CGTTGTTTTGATCGCGCCCC-ATTACGTTTGGTGGACCCTC | ||||
| nC-N1-BIP | ATACTGCGTCTTGGTTCACCGC-ATTGGAACGCCTTGTCCTC | ||||
| nC-N1-LF | TGCGTTCTCCATTCTGGTTACT | ||||
| nC-N1-LB | TCTCACTCAACATGGCAAGGAA | ||||
| nC-N2-F3 | (28285-28486) | TGGACCCCAAAATCAGCG | 202 | RT-LAMP1 | This study |
| nC-N2-B3 | GCCTTGTCCTCGAGGGAAT | ||||
| nC-N2-FIP | CCACTGCGTTCTCCATTCTGGT-AAATGCACCCCGCATTACG | ||||
| nC-N2-BIP | GGCGCGATCAAAACAACGTCG-TGCCATGTTGAGTGAGAGC | ||||
| nC-N2-LF | TTGAATCTGAGGGTCCACC | ||||
| nC-N2-LB | GCCCCAAGGTTTACCCAAT | ||||
| nC-N3-F3 | (28736-28951) | GCTGCAATCGTGCTACAACT | 216 | RT-LAMP2 | This study |
| nC-N3-B3 | TCTGTCAAGCAGCAGCAAAG | ||||
| nC-N3-FIP | TGCGACTACGTGATGAGGAACG-TTGCCAAAAGGCTTCTACGC | ||||
| nC-N3-BIP | TTCAACTCCAGGCAGCAGTAGG-CAAGAGCAGCATCACCGC | ||||
| nC-N3-LF | AGGCTTGACTGCCGCCT | ||||
| nC-N3-LB | ACTTCTCCTGCTAGAATGGCT |
aTarget gene positions are based on SARS-CoV-2 reference genome (NC_045512).
bRT-LAMP reaction by Baek et al. for technique initial setup and comparison (22)
Reverse Transcription PCR and Nested PCR
RNA extracts already were isolated according to the manufacturer's protocol for real-time PCR examination on clinical specimens with defined cycle threshold (Ct) value. The remaining RNA extracts were applied anonymously for RT-LAMP and technique validation. Synthesis of cDNA as template in nested-PCR were also carried out using the WizScript™ RT Master kit (Wizbio, Korea) according to the manufacturer's protocol, and oligo-dT in a 20 μL reaction volume.
Nested PCR reactions were performed according to PCR protocols in a volume of 50 μL, using a 2 μL RT reaction mixture, 1.0 mM MgCl2, 0.2 mM of each dNTP, 0.04 μM each set of the primer pairs (Table 1), and 1.5 units Taq DNA polymerase (Bioline). Nested PCR was performed initially by first PCR reaction and denaturation for 2 minutes at 94°C, followed by 35 cycles consisting of 30 seconds at 94°C, 45 seconds at 54°C and 3 minutes at 72°C, and one cycle of final extension of 5 minutes at 72°C. The second PCR temperature cycling conditions was the same using internal primers at an annealing temperature of 55oC. The PCR products were then visualized on agarose gel, purified, cloned and sequenced. The 422bp band then inserted into the BamHI/SalI site of pUC57_T7_CoV2a (p1) according to standard protocols as described by Fallah et al. 2003.
RT-LAMP Reaction
In a 25μL RT-LAMP reaction mixture with 1x buffer (NEB,UK) initial reagent concentrations of 0.6 mM dNTP, 4mM MgSO4, 0.6 mM betaine, 40 pmol FIP and BIP primers, 20 pmol LF and LB primers and 5 pmol F3 and B3 primers were used with 8 units of Bst 2.0 Warmstart® DNA Polymerase (NEB), 15 units of Warmstart® Rtx Reverse Transcriptase (NEB) and 5μL RNA incubated at 63oC for one hour. The transcript RNA as positive control was added in a separate room far from where RT-LAMP reagents were prepared. The LAMP products were run on an agarose gel with DNA and visualized UV transilluminator. In addition, 1μL of 10,000X SYBR™ Gold Nucleic Acid Gel Stain (Invitrogen, US) was diluted in 250μL distilled water and 1μL of the RT-LAMP reaction was added to 50μL of diluted fluorescent dye, mixed and observed under UV light.
RT-LAMP Optimization
Optimizing of RT-LAMP condition examined using different concentrations of dNTP (0-1.6 mM), MgSO4 (2-10 mM), inner and outer primers (1:2-1:10), betaine (0-1.2 M) at 61-67 oC for 15 to 75 min.
RT-LAMP Assay on Clinical Remaining Extracted RNAs
Following RT-LAMP condition optimization with artificial RNA synthesized transcribed in vitro in the presence of gene constructs as template, further evaluation was conducted using remaining RNA left from clinical specimens already processed by Real-time PCR with available defined cycle threshold (Ct) value (Table 2). Positive control (PC) and negative control (NTC) were used in each experiment.
Assessment of Limit of Detection of RT-LAMP Assays
The limit of detection (LOD) of RT-LAMP assays was determined using serial dilutions of gene construct p1 and p2 at 25 and 44 ng/mL of synthetic RNA respectively. The dilutions of 10-5,10-6,10-7 were correlated to 103,102 and 101 copies of RNA which were prepared in triplicate for LOD assessment examining with SYBR™ Gold and gel electrophoresis.
Gene constructs design
RT-LAMP primer specificity analyses
The close relative of SARS-CoV-2 is SARS-CoV. The alignment of target gene regions of these two viruses shows considerable mismatches exists between SARS-CoV-2 RT-LAMP specific primers binding site and SARS-CoV genome (Figure 2). These mismatches are higher for MERS, and Human Coronaviruses HKU1, NL63 and OC43 (data not shown).
Figure 1. Gene construct design for RNA transcript in vitro. A) Gene construct pUC57_T7_CoV2a (p1) with partial N gene sequence (28285-28529) B) Gene construct pUC57_T7_CoV2b (p2) with partial N gene sequences (28285-28529 and 28533-28758) C) Synthetic RNA transcript (1: p1, 2:p2).
Figure 2. Sequence alignment of SARS-CoV-2 with SARS-CoV. Primers for RT-LAMP-1 RT-LAMP-2 have been shown. Bule dash lines related to nested PCR amplicon 1 (see Table 1 for more details).
RT-LAMP optimization
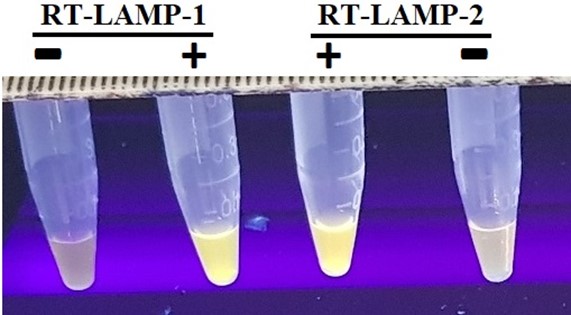
The optimum reagents concentrations including dNTP, MgSO4, betaine and primer concentrations as well as temperature and incubation time were determined for both RT-LAMP reactions 1 and 2. The optimum condition was the same for both reaction except incubation time of 35 and 30 min for RT-LAMP-1 and RT-LAMP-2 respectively. The 1.4mM dNTP, 8mM MgSO4, 0.8 M betain, 40 pmol inner primers, 20 pmol loop primers and 5 pmol outer primers with 8 units of Bst 2.0 Warmstart® DNA Polymerase and 15 units of Warmstart® Rtx Reverse Transcriptase at 65oC was obtained as optimized condition (Figure 3).
Figure 3. RT-LAMP assays 1 and 2 carried out on gene construct p2 containing both N gene fragments using different set of primers nC-N2 and nC-N3 respectively. (+): with p2 gene construct, (-): NTC.
RT-LAMP assay on synthetic RNAs from gene constructs as template
Two RT-LAMP assays were examined on 28 synthetic RNA samples including serially diluted samples. Distilled RNase free water was used as negative control (NTC). For p1 as first gene construct, target gene correlated to RT-LAMP and real time RT-PCR, were different so amplification with their specific primers occurred independently. E gene amplification by real time real time RT-PCR was excluded. The second gene construct p2 contained a fragment of target gene of N gene in common which led both RT-LAMP and RT-PCR to amplify the related amplicons with their set of primers (Table 2, Figure 1).
Table 2. Evaluation of synthetic gene constructs using real time RT-PCR and RT-LAMP gene specific primers
| Gene Construct | Gene | real time RT-PCR | RT-LAMP |
| pUC57_T7_CoV2a (p1) | N (28285-28529) | - | + |
| RdRp | + | ND | |
| pUC57_T7_CoV2b (p2) | N (28285-28529) and (28533-28758) | + | + |
| RdRp | + | ND | |
| NTC | N | - | - |
| RdRp | - | - |
NTC: No Template control
ND: Not done
RT-LAMP assay on clinical remaining extracted RNAs
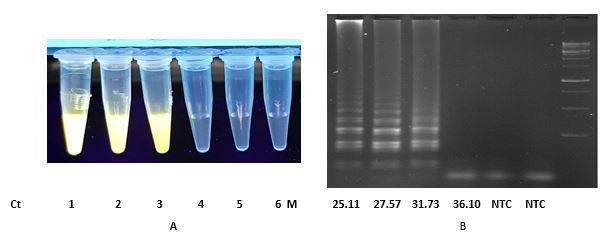
The first set of clinical samples were collected and examined in May 2021 with RT-LAMP assays 1 and 2. The second set of specimens were studied in January 2022 with RT-LAMP assays only (Table 3). To date, the in silico analysis had showed that the primer sequences for RT-LAMP 2 still fully aligned with all variant being monitored (VBM) and variant of concern (VOC) of the virus (23). Further investigation required using target genes sequencing if unexpected negative results are reported.
Table 3. SARS-CoV-2 RT-LAMP assay specificity results for synthetic and clinical samples.
| Sample type | Reaction | No. of Samples | RT-LAMP | qRT-PCRf |
| Synthetica | 1 and 2 | 28 | 28 | 13.23-32.57 |
| NTCb | 1 and 2 | 14 | - | > 40 |
| Clinical 1c | 1 and 2 | 20 | 20 | 14.06-32.57 |
| Clinical 1 | 1 and 2 | 18 | - | > 37 |
| Clinical 2d | 2 | 33 | 33 | 13.23-32.57 |
| Clinical 2 | 2 | 20 | - | > 37 |
| NTC | 2 | 10 | - | > 40 |
aThe samples tested on RT-LAMP assays 1 and 2.
bNTC: No template control
cThe first set of samples collected by May 2021 and tested on LAMP 1 and 2 assay.
dThe second set of samples collected by January 2022 and tested on LAMP 2 assay.
e Number in parenthesis showed positive result
fThe Ct values of the samples initially processed with qRT-PCR
Assessment of limit of detection of RT-LAMP assays
Two RT-LAMP assays were examined for their limit of detection on 25ng of gene construct p1 and 44ng gene construct p2.
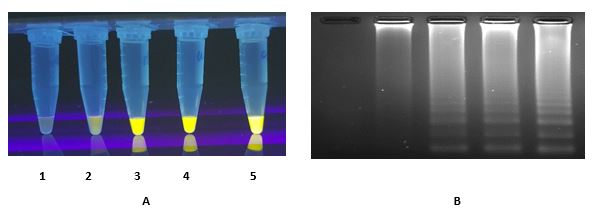
RT LAMP reaction 1 was carried out using nC-N2 primer set for N gene from nucleotide 28285 to 28486 (Table 1, Figure 4). RT LAMP reaction 2 was performed using nC-N3 primer set for N gene from nucleotide 28736 to 28951 and the ladder like bands with inverted-repeat structures were observed on agarose gel as well as SYBR Gold detection. (Table 1, Figure 5).
Figure 4. Determination of detection limit for RT-LAMP reaction 1 related to N gene fragment (28285-28529) using nC-N2 primer set. A serial dilution was prepared from 25 ng/mL (equal to 108 copy/μL) synthetic RNA. (A) SYBR Gold detection, (B) Gel electrophoresis detection. M) 1Kb marker 1) 104 copies 2) 103 copies 3) 102 copies 4) 101 copies RNA 5) No Bst enzyme, 6) No template control (NCT).
b) LAMP reaction 2
Figure 5. Determination of limit of detection for RT-LAMP reaction 2 related to N gene fragment (28285-28529) using nC-N3 primer set. A serial dilution was prepared from 44 ng/mL synthetic RNA. (A) SYBR Gold detection, (B) Gel electrophoresis detection. 1) No template control (NCT), 2) 101 copies, 3) 102, 4) 103, 5) 104 copies of synthetic RNA.
Given the rapid spread of SARS-CoV-2 and its newly emergence variants, new diagnostic approach can be provided for cutting of the transmission chains of the virus. RT-LAMP is amongst the reliable new generation molecular techniques which are simple and field applicable. When compared to real-time PCR as gold standard method, LAMP does not require specialized and expensive equipment with highly trained lab users making it a fast and affordable diagnostic option in a pandemic disease outbreak.
As part of the amplification composition, nucleic acid templates of the disease agent must be available to let the molecular technique be setup. So, the design of positive controls can respond to this urgent need to let the technique be promptly setup and optimized especially when access to the clinical specimen is a restricted issue including newly emerged variants. Compared to routine PCR, RT-LAMP technique setup is more time consuming because of uses of using six primers applied and more amplification parameters required to be optimized. Positive controls have been used individually in setting up for RT-LAMP or real-time RT-PCR assays in different studies (15, 24-27).
Different target genes were used for development of SARS-CoV-2 RT-LAMP assays. From SARS-CoV-2 genome, non-structural RdRp gene and structural genes E and N genes and their specific primers have been recommended by World Health Organization (WHO) and US Centers for Disease Control and Prevention (CDC) (19) in real-time PCR assay. To our knowledge, this is the first report that a feasible gene construct design approach is applied simultaneously as positive control in RT-LAMP for SARS-CoV-2 detection that is applicable in real-time PCR with three reference gene regions including RdRp, E and N.
Cho et. al (2020) were used different approach in designing the positive controls as templates for SARS-CoV-2 RdRP, E, and N gene expression level and only for real-time PCR (26). Because of no initial access to COVID-19 samples, Song et al. (2020) first setup an RT-LAMP assays using SARS-CoV-2 ORF1ab and N gene regions and the tests then examined and optimized on available clinical specimens in single- and two-stage detection system (28). Lamb et. al (2020) also used COVID-19 ssDNA control fragment synthesized from the nonstructural protein 3 coding region of ORF1Ab (29). The conserved regions of N gene were also used in several RT-LAMP assay setup (30, 31). N gene has been used as a good candidate for RT-LAMP assay design because of conserved regions and high levels of sub-genomic RNA expressions in clinical samples. Lu et al. (2020) developed an RT-LAMP reaction within 40 min for visual detection when more than 200 copies per 25 μL RNA were applied (31).
Baek et al. 2020 used synthetic gene for N in an RT-LAMP assay as well as examining the clinical samples and reported the detection limit of 100 copies per reaction (32). This study and designed primers were also used in our initial experiment for technique setup and evaluation. In a comparison study reexamined by Janíková et al., 2021 for several developed SARS-CoV-2 RT-LAMP assays, the limit of detection of Baek et. al works was reported 50 copies per reaction with the addition of the ribonuclease inhibitor on saliva sample (32). In our study, the detection limit of 100 copies was determined which is in the range of the patient’s viral loads (33).
For new emerging variants, SARS-CoV-2 clinical tests are mainly relied on real-time RT-PCR examinations in central testing laboratories with limited capacities and a longer time scale for test results which may cause an overload of work. In this situation, the field applicable methods such as RT-LAMP could be a solution for screening individuals and clinical specimens.
Here we reported newly constructed genes which simultaneously can be applied in both RT-LAMP and RT-PCR assays. Constructed genes with simultaneous determination capability via RT-LAMP or RT-PCR assays would be an advantage while more reliable and accurate results could be validated in technique setup.
Here we set up RT-LAMP assays using gene construct design and suggested it as a prompt approach where clinical samples are not initially available for technique establishment. These constructed genes which simultaneously can be used as positive control in standard RT-PCR can include other target genes of choice to be inserted at MCS position for new setting up of RT-LAMP assays and in vitro RNA synthesis.
This project was carried out in Iranian Research Organization for Science and Technology (IROST), Tehran, Iran under project number 1012199002. The work on synthetic RNAs and RT-LAMP setup and optimization were performed at IROST and validation of the results with remaining RNA specimens and defined Ct was managed by Lister Laboratory of Microbiology, Tehran, Iran which all supports are much appreciated.
All ethical standards had been respected.
This work was partly supported by the Ministry of Science, Research and Technology (MSRT) of I.R. of Iran and with coordinator responsibility and review of the National Institute of Genetic Engineering and Biotechnology (NIGEB).
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2022/08/18 | Accepted: 2022/09/28 | ePublished: 2023/06/26
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |