BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1808-en.html


 , Maryam Khosravy2
, Maryam Khosravy2 

 , Ali Asghar Saeedi3
, Ali Asghar Saeedi3 

 , Maryam Asli3
, Maryam Asli3 

 , Shahriar Sepahvand4
, Shahriar Sepahvand4 

 , Mohammad Darvishi5
, Mohammad Darvishi5 


2- PhD of Microbiology, Besaat Hospital, Aja university of Medical Sciences, Tehran, Iran
3- Assistant Professor, Infectious Diseases and Tropical Medicine Research Center (IDTMRC), Aja university of Medical Sciences, Tehran, Iran
4- Department of Microbiology, Falavarjan Branch, Islamic Azad University, Isfahan, Iran
5- Associate Professor, Infectious Diseases and Tropical Medicine Research Center (IDTMRC), Aja university of Medical Sciences, Tehran, Iran ,
Nosocomial infection control is now a global priority (1). The increase in hospitals and the emergence of emerging diseases, the increasing microbial resistance and the need for diverse medical services have made the incidence of nosocomial infections inevitable and faced the health care system of countries with a severe crisis.
Nosocomial infection is an infection that occurs in a patient after being hospitalized and is not the reason for his or her hospitalization. Generally, 21.6 out of every 1000 patients suffer from this problem. Although the main cause of these infections is not known, but in 17% of patients (2) these infections are mainly caused by Spp., Escherichia coli and Staphylococcus aureus (3, 4) and manifests itself mostly in the form of bacteremia, pneumonia and UTI.
Staphylococcus aureus and Escherichia coli are the leading cause of pneumonia and UTI, respectively (5, 6). Staphylococci are a part of the normal flora in humans, so they are commensal symbiotic microorganisms, but they can act as opportunistic pathogens and cause a wide range of diseases due to their rapid adaptation to selective pressures created by the host. One of the most important strategies to prevent the development of staph infections is timely diagnosis and treatment of the infections. Nosocomial infections caused by S. aureus are associated with a high mortality rate. Therefore, proper treatment of infections and determination and knowledge of the regional pattern of resistance in selecting the appropriate drug can play a key role in controlling this pathogen (7).
Clindamycin from the antibiotic class of Macrolide-lincosamide-streptogramin B (MLSB) is the most widely used antibiotic in the treatment of infections caused by S.aureus. It is also widely used in the treatment of skin, soft tissue and bone infections caused by S. aureus. There have been reports of treatment failures with clindamycin and lincomycin in the treatment of various infections caused by S.aureus due to induced resistance to these antibiotics (8-10). Widespread use of MLSB antibiotics has led to an increase in resistance to these antibiotics especially clindamycin, amongst staphylococcal strains.1, 2, 3 Macrolides such as erythromycin, roxithromycin, clarithromycin and lincosamides such as clindamycin and lincomycin belong to different classes of antimicrobials but act through the same mechanism that is by inhibition of protein synthesis. Clindamycin has long been an option for treating both methicillin-susceptible S. aureus (MSSA) and methicillin resistant S. aureus (MRSA) infections (11). Prevalence of induced clindamycin resistance in S. aureus is currently increasing (8, 12). This resistance in S. aureus varies depending on the geographical location, even the incidence of this type of resistance differs from hospital to hospital, depending on the pattern of erythromycin consumption in each region and each country. Bacteria with induced resistance are resistant to erythromycin and sensitive to clindamycin in vitro (13).
Overuse and indiscriminate use of antibiotics by humans, use in the treatment of viral infections, incomplete course of treatment, use of single drug regimens instead of multidrug regimens are among the most important factors for the rapid spread of antibiotic resistance in today's community (14). Due to the rapid spread of antibiotic-resistant staphylococci, the development of new antibiotics, finding techniques to prevent induced resistance, identifying resistant strains and how to combat them are among the most important research topics in this field. Therefore, this study aims to investigate the prevalence of clindamycin-resistant S.aureus and the resistance rate of this species to other antibiotics.
This cross-sectional descriptive study was conducted on patients suspected of having S.aureus infection referred to Beâsat Hospital, Aja University of Medical Science, in Jan. 2019 to Jan. 2021, Tehran, Iran. Non-random sampling was performed in accordance with the principles of sampling and the samples were immediately transferred to the laboratory. All samples were cultured on EMB and blood agar media and heated at 37°C for 24 h.
Inclusion criteria: Having a positive sample culture for S. aureus, including wound, sputum, blood, peritoneal fluid, urine, intravascular device, and skin, patients visiting Beâsat Hospital from 2019 to 2021. Exclusion criteria: The culture of the sample was not positive for S. aureus.
2-1. Storage of Isolated Samples
Following 16-24 h of sample culture in blood agar media, a colony was sterilized and inoculated in 7 mL of LB Broth media and cultured for 4 to 5 h at 150 rpm at 37°C on an incubator shaker to reach an OD of about 0.5 at the wavelength of 595 nm. Then, 3 mL of 100% sterile glycerol was added to the tube under the laboratory hood and completely homogenized. Finally, it was divided into 0.5 mL microtubes and quickly transferred to a -20°C freezer.
2-2. Bacterial Identification and Differential Tests
A spread of pure bacterial culture medium should be prepared on the slide prior to staining, followed by hot staining steps. Differential tests of mannitol salt agar (PadtanTebm Iran), coagulase test, catalase test and DNase test were used to identify bacteria grown on blood agar media. To that end, colonies grown were removed on blood agar media, cultured on mannitol salt agar media in four stages, and incubated for 48 h at 35°C. The yellow colonies surrounded by a yellow halo were identified as staphylococci (E.coli was used as a negative control). A drop of diluted citrate plasma was then poured onto the slide by physiological serum and added to the target colonies. After 15 to 20 seconds of incubation at 35-37°C, a microbial sample was transferred from the center of a colony to the surface of a glass slide using a wooden applicator. A few drops of 3% hydrogen peroxide (H2O2) were added to it and mixed. On DNase agar medium, the bacteria were cultured in a streaking form and heated at 37°C for 24 h. The surface of DNase agar was then coated with 1 N hydrochloric acid (HCl) by a pasteurizer pipette.
2-3. Determining Antibiotic Sensitivity
Sensitivity was determined by disk diffusion technique based on CLSI, Autoverification of Medical Laboratory Results for Specific Disciplines. 1st ed. CLSI guideline AUTO15. Wayne, PA: Clinical and Laboratory Standards Institute; 2019. Antibiotic discs were prepared from PadtanTebm, Iran including cefoxitin (30 μg), cefotetan (30 μg), cefuroxime (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), vancomycin (25 μg), co-trimoxazole (25 μg), clindamycin (25 μg), ampicillin (10 μg), oxacillin (10 μg), erythromycin (10 μg), rifampin (10 μg), trimethoprim (25 μg), and ofloxacin (5 μg). Antibiotic discs were placed on the culture media following the Lawn culture on the plate surface using sterile forceps. The plates were then cultured for 24 h at 37°C. The diameter of the growth inhibition zone was measured in millimeters using a ruler and the sensitivity of bacteria to antibiotics was then reported as sensitive, semi-sensitive and resistant. S. aureus (MRSA) ATCC 33592 was used as the standard strain. The mean and standard deviation were used, also the categorical variables were described as frequencies (%).
2-4. Data Analysis
All the data were analyzed in IBM SPSS version 21.0 (SPSS Inc., Chicago, IL., USA) and GraphPad Prism version 6 (La Jolla, CA, USA), then compared, utilizing the chi-square test. Mann-Whitney U test was used to compare quantitative character.
Of the 117 subjects, 55 were female and 62 were male. The mean age of the patients was 50.1±9.9 years (range, 10–90 years).
Most strains of S.aureus were isolated from the wound (35%) followed by blood culture (21%), urine culture (18%), sputum (16%), peritoneal fluid (8%) and catheter (2%), respectively.
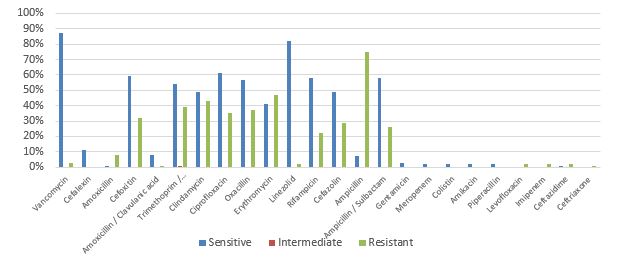
The prevalence of clindamycin resistance in the isolated samples was 48% according to the results of clinical trials. The highest resistance in this study was observed in ampicillin, followed by ampicillin/sulbactam and erythromycin (Figure 1).
Figure 1. Prevalence percentage of antibiotic resistance in Staphylococcus aureus
Furthermore, it was found that 57% of S.aureus isolated from the wound location were resistant to clindamycin. As well as 51% of isolated samples from blood were found to be sensitive to clindamycin (Figure 2).
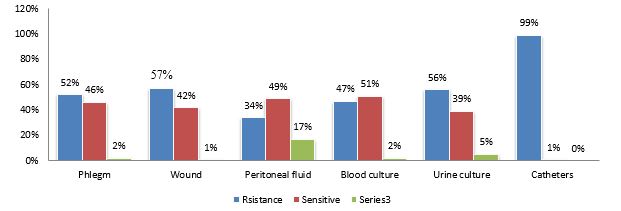
Figure 2. Prevalence percentage of clindamycin-resistant Staphylococcus aureus based on sampling locations
Overuse and indiscriminate use of antibiotics has led to the emergence of infections with a variety of resistance. Nowadays, nosocomial infections are a problem in all human communities, even in developed countries. The application of invasive methods in the diagnosis and treatment of diseases, on the one hand, has saved the lives of many people and, on the other hand, has led to deadly consequences by creating severe and resistant nosocomial infections. Nosocomial infections result in increased morbidity and mortality rate, cost, and length of hospital stay (15, 16). In this study, the most isolated samples were from wounds of those with diabetes.
In the present study, antibiotic-resistant S.aureus revealed that clindamycin is one of the appropriate antibiotics in the treatment of staphylococcal infections, especially skin and soft tissue infections. The resistance phenomenon to this antibiotic is likely due to both structural and induced mechanisms. Structural resistance can be detected using conventional disk diffusion methods. To evaluate the resistance to clindamycin, it is important to know the type of resistance, as isolates with induced resistance may mutate and become structurally resistant isolates. In this study, 43% of the statistical population had induced resistance to clindamycin. This resistance has been reported in several studies from 7% to 94% and can vary in different geographical locations and even from hospital to hospital due to differences in antibiotic prescribing (12, 13). For example, in a study by Fiebelkorn et al. (17), of 114 erythromycin-resistant S.aureus isolates, 33 isolates showed inducible resistance to clindamycin (17). Shojae et al. (13) reported 7.9% induced resistance to clindamycin (13). After examining 128 samples of S.aureus by disk diffusion method, Steward et al. (18) observed 38 cases of inducible resistance to clindamycin and according to a cross-sectional study by Marr et al. (19) in the United States, 11% of isolates had antibiotic clindamycin resistance induced with macrolide resistance (19).
Of 117 isolates, 45 isolates were resistant to erythromycin and clindamycin in our study. Moreover, 57 isolates showed simultaneous resistance to ampicillin and clindamycin. The different patterns of antibiotic resistance of the isolates can be attributed to the various geographical locations, the quality of the antibiotic discs used in different studies, the accuracy of the tests, and the reading of the results. The result of this study shows a high rate of inducible resistance in S.aureus, especially ampicillin-resistant isolates. This indicates that this type of inducible resistance is prevalent, and clindamycin can be selected as an appropriate medicine for treatment by using D-ZONE test. As a result, it is necessary to use the D-ZONE test to accurately assess the sensitivity of S.aureus isolates due to the disability of conventional antibiogram methods to identify this type of resistance.
Eventually, further studies with greater sample sizes and the combined use of herbal and chemical antimicrobial sources on other species causing morbidity and mortality are recommended to increase our knowledge of bacterial resistance.
This study revealed that inducible resistance to clindamycin is increasing, which greatly indicates the importance of determining the prevalence of induced resistance in identifying the pattern of antibiotic resistance. Preventing the indiscriminate and arbitrary use of the antibiotic clindamycin and prescribing the drug according to the results of tests for the successful treatment of S.aureus infections can prevent the development of induced resistance.
This experimental study was approved by the Ethical Committee of Islamic Azad University of Medical Sciences, Tehran, Iran (IR.IAU.TMU.REC.1397.071).
Not applicated.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of Interest
The authors declared no conflict of interests.
Received: 2022/08/5 | Accepted: 2023/01/27 | ePublished: 2023/03/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |