BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1806-en.html
Hepatitis C Virus (HCV) infection is prevalent in the United States (US), representing a serious public health threat with long-term consequences. According to recent epidemiological data, HCV seroprevalence continues to rise, particularly in the Eastern Mediterranean and European regions, with a global prevalence of 2.5% (1, 2). Egypt has one of the highest prevalence rates of HCV worldwide, with approximately 0.8 to 6.8 new cases per 1000 population every year (3). Based on viral genome sequences, there are currently seven recognized genotypes of HCV, though HCV genotypes 1 and 3 are the most prevalent worldwide. In the Middle East, genotype 4 is the most frequent (1, 4). Although HCV infection is mainly asymptomatic in the acute stage, chronic HCV is associated with a significant risk of mortality and liver-related complications (5). According to recent research, up to 30% of people with chronic HCV have liver cirrhosis, whereas decompensated liver failure affects 11% of patients (6); Furthermore, current research indicates that chronic HCV is a significant risk factor for the development of hepatocellular carcinoma (HCC) (7). Additionally, current research indicates that chronic HCV is a substantial risk factor for HCC (6).
Previously, a combination of pegylated interferon and ribavirin was the standard of care for chronic HCV, with low rates of sustained virological response (SVR) (less than 50%) and significant rates of serious side effects (8). Direct-acting antivirals (DAAs) have changed the treatment and results of chronic HCV over the last decade. DAAs are three types of medicines that work by inhibiting the three nonstructural (NS) proteins at the C-terminus of the HCV genome (NS3/4A, NS5A, and NS5B) (9). DAA medications, with or without ribavirin, exhibited a well-tolerable safety profile with high efficacy; according to current published literature, all oral DAAs for chronic HCV genotypes 1 and 3 achieved high cure rates of almost 90% in routine clinical practice settings (10, 11). In patients with or without compensated cirrhosis, a daily combination of ledipasvir 90 mg and sofosbuvir 400 mg for 12 weeks showed high SVR rates in real-life studies and is recommended (12).
However, the concept of assessing the efficacy of DAA by SVR has been criticized recently, especially after the introduction of new techniques which consider HCV genome replication in peripheral blood mononuclear cells (PBMCs) and the other different extrahepatic compartments (13, 14). The presence of HCV-RNA in PBMCs or different extrahepatic compartments in patients who achieved SVR is referred to as occult HCV infection. Patients with occult HCV are more likely to relapse and progress. However, previous studies on the frequency of occult HCV infection in individuals who underwent DAA produced mixed results (15).
Chronic HCV infection, conversely, is linked to aberrant immune responses that favour proinflammatory cytokines and chemokines upregulation (16). This altered immune response may place patients at increased risk of HCV-related consequences, especially HCC, even after virologic clearance (17). However, there is still no consensus on the influence of DAA regimens on HCV patients' immune responses.
In terms of cytokines, the evidence showed a strong link between the severity of HCV infection and CD8+ T cells, with CD8+ T cell depletion delaying HCV-RNA clearance (18). Furthermore, CD4+ cell decrease was linked to the persistence of HCV infection (19). Therefore, many studies suggested that HLA associations play a significant role in developing HCV infection, including occult cases (18-20). Besides, the elevation of serum levels of IL-4, IL-10, IFN-α, IFN-γ, and TNF-α in occult patients was observed by many investigators (18, 21, 22).
As a result, we carried out this study to determine the incidence of occult HCV infection and its relationship to the immune response of HCV patients who obtained SVR following DAA treatment.
We confirm that the current study complied with the principles of the Declaration of Helsinki and all applicable local regulatory requirements. Misr University for Science and Technology (MUST) university hospital's local ethics and research committee approved the study's protocol. Prior to research enrollment, each eligible patient signed a written informed consent form.
Study Design, Setting, and Patients:
From March to September 2019, a cross-sectional study was undertaken at MUST university's gastrointestinal department outpatient clinics. We recruited 31 adult participants with chronic HCV infection who achieved SVR12 after a 12-week ribavirin regimen of sofosbuvir 400 mg + daclatasvir 60 mg once daily patients were included regardless of how well they responded to treatment. Exclusion criteria for the study included patients with decompensated liver cirrhosis, a history of alcohol intake, and/ or a history of illicit drug use, anti-inflammatory medicines, cholesterol-lowering drugs, and other potentially immune-modifying substances. In addition, pregnant ladies were also excluded from the study. We used a non-probability consecutive sampling strategy to find eligible participants.
Data collection
Starting from March, all the studied participants had their demographic information, serum aspartate aminotransferase (AST, IU/L), serum alanine aminotransferase (ALT, IU/L), serum albumin, serum bilirubin, serum alpha-fetoprotein (AFP), serum interleukin (IL)-6, IL28B, and nuclear factor kappa B (NFK) tested.
Detection of HCV-RNA in plasma and PBMCs by quantitative real-time PCR
The manufacturer's method was followed to extract viral RNA from thawed plasma using the QIAamp1 Viral RNA Mini-Kit (cat#1048147, QIAGEN1, Qiagen, Hilden, Germany). We used the HCV Genotype 4 Qiagen as a primer for the study. First, PBMCs were separated from blood using Ficoll-Paque PLUS (Lonza, Verviers, Belgium), then lysed with the lysis buffer provided in the kits, and automated total RNA extraction from lysed PBMCs was performed using QIAamp1 RNA Blood Mini-Kits (cat#52304, QIAGEN1, Qiagen) according to the manufacturer's protocol.
Analysis of Chemokines and Cytokines
From April to July, blood samples were taken using a suction-collecting apparatus after venipuncture. Each patient received a 5 mL tube filled with gel-free anticoagulant (BD SST® Gel Advance®, Franklin Lakes, New Jersey, USA) to collect serum for CBA of circulating cytokines. The cytokines (IL-6, IL-28B, and NFK) were examined using the CBA technique and the BDTM Human Chemokine and BDTM Human Th1/Th2/Th17 Cytokine Kits, as directed by the manufacturer. IL6 levels were typically 5 to 7 pg./mL, IL-26B levels were 1.56 to 100 pg/mL, and NFK levels were 6 to 600 g/mL.
Statistical Analysis
From August to September, we used Microsoft Excel 2007 and SPSS version 22 for Microsoft Windows for data entry, processing, and statistical analysis. Quantitative data were represented by the mean with standard deviation (SD) or median with interquartile range (IQR) , while qualitative data were described by frequencies (number of cases) and relative frequencies (percentages). The unpaired Student's t-test was used for parametric data, and the Mann-Whitney Rank Sum test for non-parametric data to compare quantitative variables. The chi-square test was used to test categorical variables. Statistical significance was defined by a probability value (P-value) of less than 0.05.
Regarding the levels of cytokines of the included patients, the median IL-6 was 6.56 (5.7 – 7.9) pg./mL, and 19 % of the patients had high serum IL-6 levels. None of the patients had abnormal serum levels 28b. in addition, the median serum Nfkβ was 232 (18.7 - 609.89), and 29% had high serum Nfkβ levels (Table 2 and Figure 1).
Regarding the seroprevalence of occult HCV infection rate, the analysis showed six (19.4) patients suffering from positive occult HCV infection, and 25 patients (80.6 %) showed negative blood culture of occult HCV (Supplementary 1 Figure 1).
Patients with occult HCV infection exhibited a significantly higher values of serum IL-6 (P<0.001), Nfkβ (P<0.001), serum AST (P<0.001), serum ALT (P<0.001), serum albumin (P<0.001), total bilirubin (P<0.001), and serum AFP (P<0.001; Table 3).
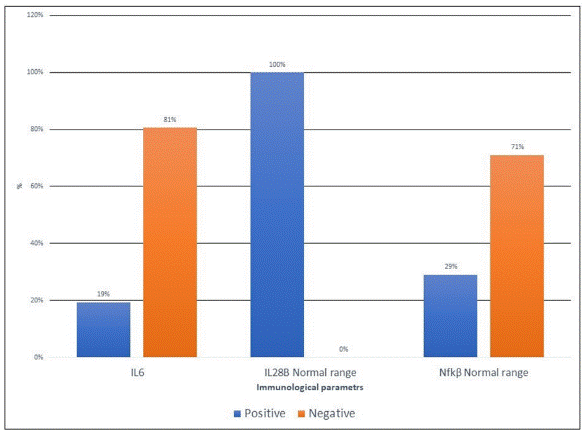
Figure 1. Bar chart of Serum cytokines of the included patients.
Table 1. The clinical characteristics of the included participants
| Variables | Patients (No. =31) |
| Age in years, Mean ±SD | 50.84 ±9.8 |
| Male, No. (%) | 16 (51.6%) |
| Serum ALT, median (IQR) | 30.5 (27.4 – 45) |
| Serum AST, median (IQR) | 25.86 (20.5 – 34.5) |
| Total bilirubin, median (IQR) | 0.86 (0.63 – 1.9) |
| Serum albumin, median (IQR) | 3.96 (3.4 – 4.3) |
| AFP, median (IQR) | 7.9 (3.9 – 55.3) |
Table 2. Serum cytokines of the included participants
| Variables | Patients (No. =31) |
| Serum IL6, median (IQR) | 6.56 (5.7 – 7.9) |
| Serum IL28B, median (IQR) | 34.31 (13 – 54.7) |
| Serum Nfkβ, median (IQR) | 232 (18.7 - 609.89) |
Table 3. The association between occult HCV infection and characteristics of the included patients.
| Variables | Positive (No. = 6) | Negative (No. = 25) | P-value |
| Age in years, Mean ±SD | 56.2 ± 9.8 | 49.5 ± 9.6 | 0.143 |
| Male, No. (%) | 4 (66.7%) | 12 (48%) | 0.42 |
| Female, No. (%) | 2 (33.33%) | 13 (52%) | |
| Serum ALT, median (IQR) | 68.17 (56.8 – 76.6) | 30.5 (27.4 – 45) | < 0.001 |
| Serum AST, median (IQR) | 74.75 (52.15 - 93.75) | 25.86 (20.5 – 34.5) | < 0.001 |
| Total bilirubin, median (IQR) | 4 (3.32 - 5.23) | 0.86 (0.63 – 1.9) | < 0.001 |
| Serum albumin, median (IQR) | 2.94 (2.45 - 3.03) | 3.96 (3.4 – 4.3) | < 0.001 |
| AFP, median (IQR) | 958.63 (652.89 - 1904.91) | 7.9 (3.9 – 55.3) | < 0.001 |
| Serum IL6, median (IQR) | 36.08 (22.7– 58.5) | 6.2 (5.6 – 6.9) | < 0.001 |
| Serum IL28B, median (IQR) | 32.64 (26.8 – 57.3) | 34.32 (9.3 – 55.6) | 0.608 |
| Serum Nfkβ, median (IQR) | 922.64 (781.6 - 993.16) | 232 (18.7 - 609.89) | < 0.001 |
As a result of significant advancements in HCV treatment, more than 90% of treated patients achieved SVR (23). However, a variety of patients reported subsequent recurrences (24, 25). After achieving the SVR (occult infection), the intracellular persistence of HCV infection was associated with later serological recurrence, including ongoing hepatocellular injury, fibrosis and cirrhosis (26, 27). In some reported cases, after initial serological clearance, replication of genomic HCV-RNA within PBMCs was accompanied by overt viremia (27, 28). Consequently, testing for HCV RNA in both serum and PBMCs at the conclusion of therapy and follow-up would be more beneficial regarding serum and cell viral RNA clearance (25).
In this cross-sectional study, the prevalence of hidden HCV in Egyptian patients who achieved SVR after DAA regimens was 19%. In the general population, the frequency of occult HCV is 3.3 %, and it is higher in those who showed complete clearance after getting sufficient medication (25, 26, 28-30). Aboalam et al. found that occult was present in 12% of PBMCs from 25 patients who had spontaneously resolved HCV infection and had a positive anti-HCV Ab serologic test but a negative blood HCV RNA PCR (30). Another Egyptian study found a significant prevalence of occult (18%) in persistent DAA responders utilizing PBMCs PCR (25). According to Yousif et al. 11.33% of patients who achieved SVR to DAAs based on negative serum HCV viremia had occult HCV. Furthermore, they found that occult prevalence varied depending on the type of treatment: 100% with SIM/SOF, 12.5% with SOF/LDVRBV, and 10.1% with SOF/DCVRBV. However, due to the study's small sample size, scientists could not link the frequency of occult HCV to a specific treatment (31). In a similar study, Abu Khadr et al. discovered a 25% frequency of secondary occult in SOV/DCV patients (32).
Castillo and colleagues found that 111 (91%) of 122 anti-HCV positive individuals who achieved SVR, defined as no HCV-RNA detectable in their serum, were positive for occult HCV infection (33). On the other hand, Mekky et al. (15) found a lower prevalence (4%) of SVR in Egyptian patients who received sofosbuvir/daclatasvir therapy. They highlighted that all of these individuals had previously failed IFN treatments. Many researchers, like Cavalheiro et al. and Radkowski et al. agreed with this conclusion and stressed the same principle in post-INF therapy (34, 35). However, it was shown that the prevalence of occult HCV was much higher post-DAAs therapy compared to post-INF treatment (36). The high variability in the incidence of occults in prior research could be owing to the sensitivity of the technologies used to detect HCV-RNA. These findings strongly suggest that occult HCV infection can exist in at least some patients who have "cleared" the virus from their serum, either naturally or during antiviral treatment.
Regarding the occult predictors, we found that positive cases are associated with significant (P < 0.001) elevation in the following parameters; ALT, AST, total bilirubin, serum albumin, AFP, serum IL6, and Nfkβ. Mekky et al. (15) also found a significant difference between positive and negative occult patients in terms of post-treatment ALT (P = 0.012), pre-treatment PT (P = 0.018), post-treatment PT (P = 0.001), pre-treatment bilirubin time (P = 0.0001), and pre-treatment albumin level (P = 0.001). Furthermore, Mekky et al. discovered that patients with a high viral load (>800,000) and pre-treatment ALT, PT, and albumin levels were more likely to have occult HCV (OR =7.03, P = 0.003; OR = 5.13, P = 0.02; OR = 2.68, P = 0.035; OR =2.52, P = 0.008). Furthermore, compared to F0, patients with F4 fibroscan results had a 4-fold increased incidence of concealed HCV (15). These findings were consistent with those of Rahman et al. and Sood et al., who found that individuals with overt cirrhosis have a higher percentage of the occult (37, 38). Yousif et al., on the other hand, found that ALT (P = 0.6), AST (P = 0.34), total bilirubin (P = 0.57), albumin (P = 0.73), INR (P = 0.22), AFP (P = 0.08), and viral load (P = 0.72) were all comparable (31).
Regarding the prospect of treating patients with occult post-antiviral therapy and the predicted outcome, no trials involving those who have completed oral DAAs (8 or 12 weeks) are currently available. In addition, there are just a few data relating to those who have had IFN-based therapy.
The flaws in the study included the lack of short- and long-term results of the included patients, as well as simultaneous testing occult in liver tissue and genetic testing. In addition, one of the study's significant drawbacks is the relatively small sample size.
The current study adds to the growing body of evidence that there is a link between occult HCV infection and decreased immune response in individuals who attain SVR after DAA treatment. Therefore, very sensitive diagnostics and novel virus isolation are needed to identify the presence of both occult and real SVR in patients undergoing these shorter treatments. Furthermore, prolonged treatment or a DAA consolidation plan may be a choice for individuals who have achieved SVR after the standard period of DAA medication but still have occult.
None.
This study was supported by Vice-Chancellor for Research and Technology of Kermanshah University.
Conflicts of Interest
No funding or grant was received for this paper.
Received: 2022/07/4 | Accepted: 2023/01/29 | ePublished: 2023/03/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








