BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1568-en.html


 , Farzin Sadeghi2
, Farzin Sadeghi2 

 , Kazem Aghajanipour1
, Kazem Aghajanipour1 

 , Ali Hasanzadeh3
, Ali Hasanzadeh3 

 , Mohammad Chehrazi4
, Mohammad Chehrazi4 

 , Yousef Yahyapour5
, Yousef Yahyapour5 


2- Cellular and Molecular Biology Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran
3- Department of Microbiology, Faculty of Medicine, Golestan University of Medical Sciences, Gorgan, Iran
4- Department of Biostatistics and Epidemiology, School of Public Health, Babol University of Medical Sciences, Babol, Iran
5- Infectious Diseases and Tropical Medicine Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran ,
The infections of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) are known as the main causes of acute viral hepatitis all over the world (1). HAV is a member of the Picornaviridae family, while HEV is in the Hepeviridae family, and both of them are non-enveloped icosahedral viruses (2, 3). The absence of a lipid envelope enhances their ability to spread by the food and waterborne environment. Also, they are transmitted via the fecal-oral way (either via close person-to-person contact or ingestion of fecal) (4, 5). Although both viruses can be spread by blood transfusion (6, 7), HEV can also be transmitted through zoonotic and vertical (maternal-fetal) routes (8). HEV is associated with a higher mortality rate compared to acute HAV infection (9). The World Health Organization (WHO) estimates the HAV infection rate of about 1.4 million cases around the world each year, which leads to 7,000 deaths approximately. In addition, there are nearly 20 million HEV infections per year and around 3.3 million persons with symptoms, and almost 44,000 deaths (10). The HAV and HEV epidemiological and transmission pattern are very different around the world that is highly linked to the socioeconomic indicators. The incidence of HAV infection has been reduced in the developed countries because of the access to better sanitation facilities, clean drinking water, and vaccination; but in poor resources and developing areas, like parts of Asia, South America, and Africa, due to poor sanitation and deficiency of clean water, the incidence rate of HAV varies from high to moderate and low in different areas (11, 12). Mostly, HAV infection in children is asymptomatic and very mild and typically leads to lifelong immunity. Unlike HAV, HEV infection does not lead to lifelong immunity, and there is a risk of re-infection when anti-HEV IgG seroprevalence drops below a critical threshold (13, 14). The seroprevalence of these viruses in Iran has been evaluated by some studies, which showed Iran as an endemic region with approximately more than 64% anti-HAV serum positivity (15-17). Also, in some HEV seroprevalence studies in Iran, there is an anti-HEV rate between 1% to 46%, which is indicative of Iran as an endemic region (18). Neighboring with countries such as Iraq and Syria that are highly endemic areas and numerous Iranians go on pilgrimage to these countries with huge crowds, are the main reasons to become susceptible to get infected with HAV and HEV (19, 20). Babol with more than half a million population is one of the most densely populous counties in the North of Iran. The seroprevalence of HEV and HAV has been evaluated in this area (21-23). However, no up-to-date community-based investigation was found reporting about HAV and HEV seroprevalence in this region. The present investigation was designed to assess the HAV and HEV seroprevalence in blood donors in Babol to figure out the infection and immunity incidence rate of these viruses in the population. This unambiguous and updated knowledge of the HAV and HEV seroprevalence is essential to evaluate the impact of prevention and control measures including vaccination strategies.
Study population
This cross-sectional study was conducted on blood donation volunteers referred to the blood transfusion centers of Babol county, North Iran, between July to December 2018. A total of 491 blood samples were collected from blood donors with no history of HBV, HCV, HIV, and chronic liver diseases. Donors' information, including social and demographic data (gender, age, urban/rural residence status, education, and marital status) was collected by a standardized questionnaire for analysis. This study was accepted by the ethical committee of Babol University of Medical Science, and written informed consent was obtained from all subjects (Ethics Committee code: IR.MUBABOL.REC.1397.044)
Laboratory Analysis
Blood samples (5 mL) were collected from the participants and centrifuged. The separated serum was stored at -20°C until further laboratory analysis. Commercial enzyme-linked immunosorbent assay kits (DIAPRO, Diagnostic Bioprobes, Milano, Italy) were used to detect anti-HAV and anti-HEV IgG antibodies in the serum samples, according to the manufacturer's instructions. For the anti-HAV IgG ELISA kit, the detection limit and sensitivity were <0.01 IU/mL and 0.15 IU/mL, respectively. For the anti-HEV IgG ELISA kit, these values were <0.1 IU/mL and 0.25 IU/mL, respectively.
Statistical Analysis
The SPSS software v. 16.0 (IBM, Chicago, IL, USA) was used for the statistical analysis. The anti-HAV/HEV seroprevalence in the stated stratification factors was analyzed using the Chi-square test. The P-values less than 0.05 were considered statistically significant.
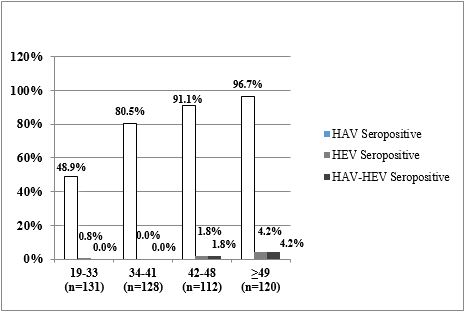
The demographic data and seroprevalence of HAV and HEV of the study participants were shown in Table 1. Of the 491 blood donors, 385 (78.4%) and 8 (1.6%) were positive for anti-HAV IgG and anti-HEV IgG, respectively. The overall mean age of blood donors was 40.92 ± 9.86 years, with a range of 19-61 years. Also, the mean ages of positive anti-HEV and anti-HAV antibodies were 49.13 ± 11.43 and 43.35 ± 8.92 years, respectively. Besides, there was a strong inverse statistical association between the marital status and anti-HAV positivity (P<0.0001). Our data showed higher HAV incidence in urban regions (P=0.29) and among the participants with education level less than high school diploma (P=0.01). Also, the rate of anti-HAV seropositivity among the age groups of 19-33, 34-41, 42-48, and older than 49 years, increased gradually (from 48.9% in the youngest age group to 96.7% in the oldest group of blood donors) (P<0.0001) (Figure 1). The co-seropositivity of HAV and HEV was observed in 5 (1%) blood donors. All the co-seropositive cases were male, but there was no statistical association (P>0.05). A significant correlation was observed between the academic levels of education compared to the high school diploma [OR= 0.47 (0.28 – 0.80; P=0.005)] (Table 2).
Table 1. Characteristics of blood donors between positive and negative anti-HAV and anti-HEV seroprevalence.
| All (n=491) | Anti-HAV n (%) | P | Anti-HEV n (%) | P | |||
| positive (n=385) | Negative (n=106) | positive (n=8) | Negative (n=483) | ||||
| Gender | |||||||
| Male | 471 | 371 (78.8) | 100 (21.1) | 0.35 | 8 (1.7) | 463 (98.3) | 0.55 |
| Female | 20 | 14 (70) | 6 (30) | 0 (0.0) | 20 (100) | ||
| Age | |||||||
| 19–33 | 131 | 64 (48.9) | 67 (51.1) | <0.001 | 1 (0.8) | 130 (99.2) | 0.16 |
| 34–41 | 128 | 103 (80.5) | 25 (19.5) | 0 (0.0) | 128 (100) | ||
| 42–48 | 112 | 102 (91.1) | 10 (8.9) | 2 (1.8) | 110 (98.2) | ||
| 49≤ | 120 | 116 (96.7) | 4 (3.3) | 5 (4.2) | 115 (95.8) | ||
| Marital Status | |||||||
| Single | 44 | 15 (34.1) | 29 (65.9) | <0.001 | 1 (2.3) | 43 (97.7) | 0.72 |
| Married | 447 | 370 (82.8) | 77 (17.2) | 7 (1.6) | 440 (98.4) | ||
| Place of Residence | |||||||
| Urban | 339 | 275 (81.1) | 64 (18.9) | 0.29 | 6 (1.8) | 333 (98.2) | 0.71 |
| Rural | 152 | 110 (72.4) | 42 (27.6) | 2 (1.3) | 150 (98.7) | ||
| Educational Level | |||||||
| Less than high school diploma | 212 | 177 (83.5) | 35 (16.5) | 0.01 | 4 (1.9) | 208 (98.1) | 0.51 |
| high school diploma | 150 | 117 (78) | 33 (22) | 1 (0.7) | 149 (99.3) | ||
| University level | 129 | 91 (70.5) | 38 (29.5) | 3 (2.3) | 126 (97.7) | ||
Table 2. Comparison of Demographic characteristics of blood donors between Positive and Negative anti-HAV.
| Characteristics | HAV | Adjusted OR (CI 95%) |
P | Unadjusted OR (CI 95%) |
P | |
| Positive, N (%) | Negative, N (%) | |||||
| Sex | ||||||
| Male | 371 (78.8) | 100 (21.1) | 1 | - | 1 | - |
| Female | 14 (70) | 6 (30) | 0.58 (0.8 – 1.86) | 0.36 | 0.63 (0.24 – 7.51) | 0.35 |
| Age | ||||||
| 19-33 | 64 (48.9) | 67 (51.1) | 1 | - | 1 | - |
| 34-41 | 103 (80.5) | 25 (19.5) | 2.99 (1.64 – 5.44) | < 0.0001 | 4.31 (2.47 – 7.51) | < 0.0001 |
| 42-48 | 102 (91.1) | 10 (8.9) | 7.11 ( 3.29 – 15.39) |
< 0.0001 | 10.68 (5.12 – 22.25) | < 0.0001 |
| 49≤ | 116 (96.7) | 4 (3.3) | 20.60 (7.01 – 60.46) | < 0.0001 | 30.36 (10.58 – 87.10) | < 0.0001 |
| Educational Level | ||||||
| Less than high school diploma | 177 (83.5) | 35 (16.5) | 1 | - | 1 | - |
| High school diploma | 117 (78) | 33 (22) | 0.85 (0.46 – 1.57) | 0.61 | 0.70 (0.41 – 1.19) | 0.19 |
| University level | 91 (70.5) | 38 (29.5) | 0.63 (0.34 – 1.17) | 0.63 | 0.47 (0.28 – 0.80) | 0.005 |
| Place of Residence | ||||||
| Urban | 275 (81.1) | 64 (18.9) | 1 | - | 1 | - |
| Rural | 110 (72.4) | 42 (27.6) | 0.61 (0.36 – 1.04) | 0.07 | 0.61 (0.39 – 0.95) | 0.03 |
| Marital Status | ||||||
| Single | 15 (34.1) | 29 (65.9) | 1 | - | 1 | - |
| Married | 370 (82.8) | 77 (17.2) | 3.25 (1.50 – 7.03) | 0.003 | 9.29 (4.75 – 18.15) | < 0.0001 |
Figure 1. Age-Specific Seroprevalence of Hepatitis A Virus and Hepatitis E Virus among Blood Donors (Northern Iran)
HEV and HAV are the important reasons for acute self-limiting viral hepatitis worldwide and the severe form of infection can lead to a significant mortality rate among pregnant women (24). According to the report of the Centers for Disease Control and Prevention (CDC), Iran is an endemic area for HAV and HEV infections (19, 25). Our study showed the overall seroprevalence of HAV and HEV in the blood donors at 78.4%, 1.6%, respectively. Some other studies on the rate of anti-HAV seroprevalence among Iranian blood donors reached similar results to our study that reported 86% and 94.9% in Tehran and Qazvin provinces, respectively (26, 27). Another study on 559 blood donors by Hesamizadeh et al., in 2016 showed 70.7% HAV seropositivity (15).
Our data showed that 1.6% of Babol blood donors (northern Iran) were positive for anti-HEV. The seroprevalence of anti-HEV in Babol county was lower than the previous reports published from other regions of Iran. Similar demographic studies from other parts of Iran are needed to map the geographical distribution of the virus for preventive decisions. Studies among the blood donors showed 7.7% from the study in Capital Iran (Tehran) (26), 7.8% in Northwest (West Azarbaijan) (28), 8.1% in Tehran (29), 11.5% in Southwest (Khuzestan) (30), and 14.3% in Central of Iran (Markazi) (31) provinces for the HEV seropositivity.
Merat et al., indicated that the anti-HAV was 85%, 96%, and 99% in Tehran, the South of Iran (Hormozgan), and Northeast Iran (Golestan) provinces (24). In 2008, Elikaei et al., (26) reported that the prevalence of total anti-HAV antibodies in 407 blood donors in Tehran Blood Transfusion Center was 86%, which means the high prevalence of total anti-HAV antibodies among the blood donors; that indicates their infection since childhood. In 2011, Ramezani et al., (27) conducted a study on 351 blood donors in Qazvin, of which 333 samples (95%) were positive for HAV IgG. These results showed a very high rate of HAV Ab among blood donors in Qazvin, which indicates a high level of contact with HAV in childhood.
In 2013, Johargy et al., (32) conducted a study on 900 male serum samples collected from four blood transfusion centers in Mecca, Saudi Arabia, of which 168 samples (7.2%) were positive for HEV IgG. These results indicate that exposure to the HEV is higher in Mecca than in the surrounding areas. In a study on 200 Serbian volunteer blood donors, 15% were HEV seropositive and the prevalence increased with age. The highest HEV seroprevalence rates were found in individuals older than 51 years (33).
In Hoseini et al. study, among the adolescent population, anti-HAV was shown to be lower than in our study because of the younger ages of the population in his study (16). Other countries' studies on HAV seroprevalence reported miscellaneous results around the world, in the Middle East and African regions like Turkey, Saudi Arabia, Algeria, and UAE that showed intermediate HAV endemicity. Morocco, Lebanon, Jordan, and Tunisia had high endemicity like in Iran. Yemen, Iraq, Palestine, Syria, and Egypt had very high endemicity (34). Areas in developed countries, like North America and European :union: (EU), are considered regions of very low HAV endemicity.
Differences in the prevalence of HAV are related to the healthy water access, sanitation, income level, and social facilities that had a direct effect on the outbreak of HAV in countries (35, 36).
Studies about HEV reported different seroprevalences in Iran. Southern regions of Iran, like Ahvaz, the center of Khuzestan, which is our border with Iraq, showed the highest anti-HEV level (46%) (28). The other regions like Tabriz and Tehran showed much lower prevalence, under 10% (22, 29). A meta-analysis study estimated the overall anti-HEV in Iran as approximately 10% (18), but our study showed a much lower seroprevalence of HEV (1.6%).
Like anti-HAV, the anti-HEV seroprevalence in Africa and Asia have shown the highest endemicity rate in the world. Europe and North America have lower rates, respectively compared to Africa and Asia. The Middle East area like Saudi Arabia, Yemen and Qatar have shown higher rates of anti-HEV compared to Iran (37).
All studies showed both HAV and HEV as public health challenges to both developing and developed countries. Changing the epidemiological and lifestyle patterns about socioeconomic progress in developing countries may help them to reduce the infection rate. Long-term and comprehensive plans like childhood vaccination programs must be properly implemented on time, especially in HAV and HEV endemic regions.
Our findings, similar to many other studies, showed that the prevalence of both HAV and HEV were not significantly associated (P>0.05) with sex and different age groups (15, 25). Our study, similar to Taghavi et al., reports (38) in Southern Iran (Fars province) and Hesamizadeh et al. (15) in Tehran showed that the level of anti-HAV and anti-HEV seroprevalence increased significantly by age, so that when the people get older, they become more exposed to both HAV and HEV.
The present study found increased prevalence of HAV by age, also 48.9% of blood donors in the 19-33 age groups were positive. Our results were in contradiction to the results of Mohebbi et al., study (25). They reported on Tehran blood donors, that participants of >47 years old had the highest rate (94.8%) and aged 18-27 years old had the lowest rate (26.7%) of anti-HAV seropositivity. This rate was 58.5% in people younger than 30 years old (25).
We noticed a significant inverse association between educational status and HAV. The HAV-positive rate increased with lower education levels (P=0.019), which was consistent with Ramezani et al. study results (27).
Outstanding to developed health situations, the risk of increasing hepatitis is reduced in infantile but increased in old age. This study showed that the incidence of HAV in the age group of fewer than 33 years is low. Also, it is critical to consider the many problems of these diseases during pregnancy. Consequently, the finding of hepatitis A and E viruses in women can be important. In recent years, improvements in hygiene, sanitation, and access to more safe and clean water in Iran, leading to the incidence rate of HAV and HEV, have decreased and increased the average age of infection. In our study, the incidence of HEV has not changed compared to the past, and HEV was lower than in other regions of Iran. Also, for more prevention and control of HAV and HEV, vaccination plans are to be decided, and it needs more investigation in the north of Iran to obtain detailed information on anti-HEV seroprevalence.
We would like to thank Babol University of Medical Sciences for funding this study (Project code: 9706019).
This study was accepted by the ethical committee of Babol University of Medical Science, and written informed consent was obtained from all subjects (Ethics Committee code: IR.MUBABOL.REC.1397.044).
Conceived and designed the analysis: Mostafa Javanian, and Yousef Yahyapour
Collected the data: Kazem Aghajanipour, Ali Hasanzadeh, and Yousef Yahyapour
Contributed data or analysis tools: Mohammad Chehrazi, Farzin Sadeghi, and Yousef Yahyapour
Performed the analysis: Yousef Yahyapour, Mohammad Chehrazi, Farzin Sadeghi, and Mostafa Javanian
Wrote the paper: Mostafa Javanian, Farzin Sadeghi, Ali Hasanzadeh, Mohammad Chehrazi, and Yousef Yahyapour.
This project was funded by the Vice-Counselor for Research and Technology of Babol University of Medical Sciences (grant No: 9706019), and this was extracted from the residency thesis.
Conflicts of Interest
The authors declare no conflict of interest.
Received: 2021/11/29 | Accepted: 2022/02/15 | ePublished: 2022/05/25
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |