BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1536-en.html

 , Hamid Staji2
, Hamid Staji2 

 , Morteza Keywanlou3
, Morteza Keywanlou3 
 , Mohammadreza Salimi Bejestani1
, Mohammadreza Salimi Bejestani1 
 , Ehsan Gallehdar Kakhki4
, Ehsan Gallehdar Kakhki4 

2- Departement of Pathobiology, Faculty of Veterinary Medicine, Semnan University, Semnan, Iran ,
3- Department of Clinical Sciences, Faculty of Veterinary Medicine, Semnan University, Semnan, Iran
4- Small Animal Veterinary Clinician, Mashhad, Iran
Anaplasmosis, a disease caused by Anaplasma spp., belongs to the complex of several arthropod-borne diseases with zoonotic potential. Anaplasma are gram-negative obligate intracellular pathogens, infecting blood cells of mammals and vertebrate hosts act as reservoirs for this microorganism. The major vectors of the Anaplasma spp. are Ixodes ticks distributed worldwide (1,2).
In the vertebrates, Anaplasma phagocytophilum, Anaplasma platys, Anaplasma capra, Anaplasma marginale, Anaplasma ovis, and Anaplasma bovis are considered as major pathogens (3–5). Likewise, species with zoonotic potential have been reported as well as A. phagocytophilum, A. capra, and A. ovis which are transmitted most often through the bite of a hard tick to humans (6,7). Depending on the Anaplasma spp., various cells in the reticuloend-othelial system as well as granulocytes, platelets, erythrocytes, and bone marrow precursor cells or endothelial cells may be infected (2), resulting in a variety of clinical signs from mild to life-threatening illness including anemia, gastrointestinal and pulmon-ary disorders, and joint pain and lameness (8). A. phagocytophilum is a frequently characterized path-ogen from small ruminants, horses, dogs, and humans, which infects granulocytes causing Granulocytic Anaplasmosis (GA), a multisystemic disease with subclinical or severe symptoms. Ixodes and Dermac-entor ticks are the major vectors for A. phagocy-tophilum (2).
The present study deals with detecting A. phagocytophilum in the blood specimens of sheltered dogs from Mashhad city in the northeast of Iran. Besides, the detected pathogens were phylogenet-ically compared to typed strains of A. phagocy-tophilum present in the GenBank.
In this investigation, 5 mL of blood specimen via the saphenous vein of 250 dogs (103 males and 147 females) belonging to different breeds and aging from 2 months up to 12 years, sheltered in Mashhad (Iran), were collected in EDTA containing tubes. Sampling was carried out during a routine health checkup program. The blood samples were placed on ice and immediately transferred to the faculty of veterinary medicine of Semnan University. Then, DNA extraction was performed on the buffy coat of blood specimens using a DNA extraction kit (Sinapure DNA whole blood, serum, and plasma, Sinaclon, Iran). The extracted DNA samples were preserved in a -20 freezer until molecular assays. Additionally, the blood smears were directly prepared and fixed by methanol. Then, fixed smears were stained by Giemsa and observed by a light microscope.
Based on the morphology of pathogens infecting granulocytes in microscopic observations, the extracted DNA samples from buffy coats were screened for the presence of Anaplasma spp. by realtime-PCR amplifying a 1400 bp fragment specific to 16S rRNA according to the protocol described by Dyachenko et al. (9) with some modifications in thermal conditions. Real-time PCR amplifications were performed using a Rotor-Gene Q MDx real-time PCR instrument (QIAGEN). The PCR reactions were performed in a final volume of 20 μL containing 2X real-time PCR Master Mix (SYBR® Green I; BioFACT™), 500 nM of primer pairs (VD2f: 5'-AGAGTTTGA-TCCTGGCTCAG-3', and VD2r: 5'-CGGCTACCTTG-TTACGACTT-3'), and 100 ng of the DNA template. In this experiment, A. platys were used as the positive control, and distilled water was used as the negative control. Data were analyzed using Rotor-Gene Q series software 2.3.1 (QIAGEN). Moreover, for the size determination of amplified fragments, realtime-PCR products (10 μL) were electrophoresed in 1.5% agarose (Sigma-Aldrich) gel, stained with ethidium bromide (Sigma-Aldrich), and visualized under a UV illuminator (Nanolytik™, England). Mid-range DNA ladders (100 bp, Jena Bioscience) were used for fragment size determination.
For phylogenetic analysis of detected Anaplasma strains as a preliminary screening, the extracted DNA from blood specimens of Anaplasma-positive dogs in previous realtime-PCR steps were subjected again to conventional PCR for amplification of the 1400 bp fragment of 16S rRNA. The reactions were prepared in total volumes of 50 μL with 15 pmol of each primer VD2-f and VD2-r (9), 25 µL of 2X PCR Master Mix (Jena Bioscience, Germany), and 500 ng of genomic DNA. After an initial denaturation step at 94°C for 60 s, the reactions were cycled 35 times (BIOER XP Cycler, China) as follow: denaturation at 94°C for 30 s, annealing at a temperature specific (achieved by gradient temperature conditions) to the primer pairs 54°C for 45 s, and extension at 72°C for 90 s. Then a final extension step was followed at 72°C for 5 min to complete the last PCR cycle. After electrophoresis of PCR amplicons on 2% agarose gel, the 1400 bp products were sent for nucleotide sequencing.
The nucleotide sequence of the amplified fragments was analyzed with an ABI 3730XL DNA Analyzer according to an automated Sanger dideoxy fluores-cent nucleotide method. The BLAST software was applied to determine the homology of the amplified fragments to 16S rRNA sequences of Anaplasma spp., existing in GenBank. The evolutionary history was inferred using the Maximum Likelihood method and Tamura-Nei model (10, 11). The tree with the highest log likelihood (-6943.31) is shown. The initial tree for the heuristic search was obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura-Nei model and then selecting the topology with a superior log-likelihood value. This analysis involved 32 nucleotide sequences. There were a total of 1575 positions in the final dataset. Evolutionary analyses were conducted in MEGA X.
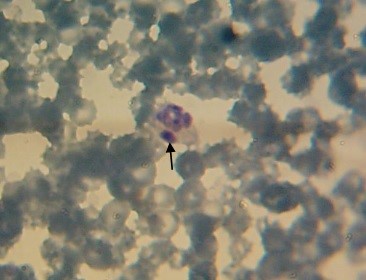
Typical large basophilic inclusions (morulae) were observed in neutrophils of 3 dogs (Figure 1) during blood smears observation. Realtime-PCR demons-trated that Anaplasma 16S rRNA was detected in 9 out of 250 (3.60%) blood specimens, including 4 (0.38%) positive specimens from male dogs and 5 (0.34%) from female dogs, respectively (Figure 2). Statistical analyses on the prevalence of Anaplasma-positive samples across dogs stratifying by gender and aging showed that the presence of Anaplasma was not significant in different genders and age groups (P>0.05).
Figure 1. Anaplasma morulae (black arrows) in the neutrophil of a dog in the present study, 4000 ×, stained by the Giemsa method.

Figure 2. Cycling Threshold (CT) of Anaplasma spp. 16S rRNA specific fragment in blood specimens from monitored dogs. Amplification of 16S rRNA in blood specimens of dogs are noticed in addition to A. platys as the positive control.
In the present investigation, the obtained seque-nces of 16S rRNA belonging to Anaplasma spp. showed genetic identities of 98.05–98.13% with the sequences of A. phagocytophilum type strains present in GenBank. The phylogenetic tree based on the Maximum Likelihood method in the present study indicates the position of our detected strains and their identity with the type strains of A. phagocytophilum and other closely related species (Figure 3). The optimal tree is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. Analysis of 16S rRNA sequence from detected Anaplasma strains infecting dogs demonstrated a high similarity level with the A. phagocytphilum strains: Norway variant2 (CP01537-6.1), Dog 2 (CP006618.1), Hubei E4(KF569909.1), JXAAGX-49 (MH722235.1), Yeyasu (LC334014.1), and HB-SZ-HGA-S05 (HQ872465.1) deposited in GenBank by other researchers (Figure 3).

Figure 3. Phylogenetic analysis of A. phagocytophilum strains identified in the present study based on the 16S rRNA sequencing. The tree was constructed using the Maximum Likelihood method. A. ovis, A. marginale, A. platys and A. capra strains were used for phylogenetic comparisons.
Anaplasma phagocytophilum, a tick-borne patho-gen, is emerging in some vertebrates, mainly humans and dogs, worldwide (12). Several predisposing factors play an essential role in arthropods' multiplication and spread, increasing the possibility of Ixodidae ticks feeding on humans and dogs and transmitting A. phagocytophilum to these hosts (13).
In Asia, the first report regarding the serological evidence of the infection with A. phagocytophilum in a human was documented in Korea (14). Then, the first Molecular report of A. phagocytophilum infection was documented by Kawahara (2006) and Ooshiro (2008) in cattle and wild deer in Japan (15,16). In Iran, A. phagocytophilum was detected by PCR-RFLP for the first time as a potential novel arthropod-transmitted agent in the Ixodes ricinus ticks (17), and subsequ-ently, it has been reported from different vertebrates and tick vectors from different parts of Iran (18–21). Molecular detection of A. phagocytophilum revealed a point prevalence of 3.6 % in our study population. Other studies carried out in Iran regarding the molecular prevalence of A. phagocytophilum in dogs found a prevalence range between 2-57% (22,23). The different ranges of Anaplasma infections in dogs from various regions can be related to the differences in the prevalence of vector ticks in these regions because factors including climate conditions and acaricide treatments can influence the spread of infected vectors (20). In the present study, A. phagocytophilum was detected in blood smears of only 3 dogs (1.2%), while molecular detection by real-time PCR identified 9 dogs (3.6%) infected with this pathogen. The different obtained results between the two applied methods (1.2% vs 3.6%) are due to the less sensitivity of microscopic observation than real-time PCR. Similar results have been observed in previous investigations (23). The "Gold standard" method for the diagnosis of Anaplasma spp. relies on the combination of the microscopic examination and cELISA. The indirect immunofluorescence antibody test is widely used to diagnose blood protozoon and Rickettsia. The IFA test is commonly used in epidemiological studies because of its low costs (24).
The primer pairs (VD2-f & VD2-r) of the real-time PCR used in this study and 16S rRNA sequencing of Anaplasma spp. have been applied effectively for genotyping and differentiation of Anaplasma spp. in previous investigations. It has been stated that these molecular methods are highly sensitive and specific for phylogenetic purposes because the sequence of 16S rRNA fragment of Anaplasma spp. has a small hypervariable region (9, 25–27). In the present survey, characterization of detected A. phagocytophilum strains by real-time PCR and subsequent 16SrRNA sequencing of strains determined them very close to type-strains Dog 2 (CP006618.1), Norway variant2 (CP015376.1), Hubei E4(KF569909.1), JXAAGX-49 (MH722235.1), Yeyasu (LC334014.1), and HB-SZ-HGA-S05 (HQ872465.1) deposited in GenBank by other researchers, phylogenetically (Figure 3). These mentioned relatively close type-strains of A. phagocytophilum to the strains of the present study have been identified from humans (China), dogs (Japan and Norway), and goats (China), previously (28–31). As our knowledge rises more about the genetic similarity of A. phagocytophilum strains from different hosts, the evidence is developing that this pathogen infects a broader range of vertebrate hosts than previously thought. Human exposure to ticks carrying Anaplasma spp. has been increased by environmental and land-use changes causing more contact between humans, animals, and vector reservoirs (31). Stray and sheltered dogs can act as a reservoir for A. phagocytophilum, infecting immature stages of Ixodidae ticks which act as bridge vectors transferring Anaplasma to humans and livestock when they are symptomatic or asymptomatic (20). These animals circulate in urban and suburban places worldwide and contact humans in public places and livestock on farms. Our results (Figure 3) confirm the fact that stray and sheltered dogs can serve as an important reservoir for different genotypes of A. phagocytophilum and can transmit it to vectors specifically to those ticks infesting other livestock and human. Whether the existence of A. phagocytophilum in sheltered dogs in Mashhad (Iran) poses a considerable zoonotic hazard for humans remains undetermined. This study should signal Iranian physicians and veterinarians that A. phagocytophilum exposure and infection are not rare, and it will help raise alertness on the potential incidence of anaplasmosis more in this region. Since we reported results in a limited area of the country and on a very limited number of dogs, larger and more represent-tative investigations are recommended, especially on human cases with anaplasmosis and genotyping of A. phagocytophilum strains identified in Iran from the human.
There seems to be a high risk of infection with A. phagocytophilum for dogs in Iran. Our findings highlight the significance of these animals as a potential hazard for livestock and humans. Besides, 9 strains of A. phagocytophilum were identified in dogs during this study using 16S rRNA sequencing. Thus, it can be concluded that more A. phagocytophilum genotypes should be expected to exist in dogs. More investigations and monitoring seem to be required in dogs and other vertebrates associated with different geographic regions in Iran to determine the epide-miologic distribution of A. phagocytophilum genot-ypes. The clinical significance of the results of this study remains to be elucidated in future invest-igations.
The authors gratefully acknowledge the staff of the Laboratory of Microbiology, Faculty of Veterinary Medicine, University of Semnan (Iran), especially Mrs. Behnaz Raeisian and Mr. Rasoul Rostami Lima.
HS: Conceived and designed the analysis; NM, MK, EGK: Collected the data; HS and MK: Contributed data or analysis tools; HS: Performed the analysis; HS, NM, MSB, MK: Wrote the paper.
This study was carried out in partial fulfillment of a DVM student's thesis requirements. We also acknowledge Semnan University for funding the study.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2021/11/2 | Accepted: 2022/01/19 | ePublished: 2022/03/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |