BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1475-en.html
2- Department of Pathology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran , m.mehdinejad1017@gmail.com
3- Department of Pathology, RASOOL-E-AKRAM Hospital, Iran University of Medical Sciences, Tehran, Iran
PBacterial durability to antibiotics is an essential scientific issue related to community health as well as hospitals, which has led to the failure of treatments for dangerous infections (1,2). Fast detection of organisms' resistance to antimicrobial is important in clinical laboratories (3). Characterization of the resistance mechanism can facilitate innovation in antimicrobials design. Three general pathways for antibiotic resistance have been identified: A) The generation of beta-lactamases, which break the beta-lactam loop and thus inactivate the beta-lactam antibiotic, B) Lack or decrease in the exposition of outer membrane proteins called OMP in gram-negative pathogens and C) Increased expression of membrane pumps (4-7). ESBL production is an important resistance mechanism that prevents the antimicrobial treatment of gram-negative infections and shows a significant hazard to available antibiotics, particularly β-lactam antibiotics, including penicillins, cephalosporins, and monobactams (8-10). AmpC β-lactamases preferentially hydrolyze narrow, broad, and expanded-spectrum cephalosporins and cepham-ycins and abide blockage by clavulanate, sulbactam, and tazobactam. AmpC-producing strains are generally resistant to oxyimino-beta lactams and cephamycins and are sensitive to carbapenems, but decreased porin expression will build such a strain carbapenem-persistent as well (11).
The introduction of carbapenems to the medical world has been the main breakthrough in the remedy of diseases caused by beta-lactam-resistant bacteria. Carbapenems are the Preferred medicine for treating infections caused by penicillin-resistant or cephalo-sporin-resistant gram-negative bacilli due to their wide range of activity and resistance to hydrolysis by most beta-lactamases (12,13). Resistance to carbap-enem has been attributed to various factors such as decreased expression of outer membrane proteins, increased efflux activity, and production of carbapen-emase beta-lactamases (14).
According to the Ambler molecular classification, beta-lactamases are divided into four groups: class A, C, and D are serine beta-lactamase, and class B is Metallo-β-lactamase (MBL) (15). Extended-spectrum Beta-lactamases are interdicted by clavulanic acid, sulbactam, and tazobactam and are encoded by different genes (including blaSHV, blaTEM, blaOXA, blaPER, blaVEB-1, and blaBES-1) that can vary between bacteria (16-19). Among the various types of ESBL, the most common subgroups are blaCTX-M, blaTEM, and blaSHV, which are crucial factors in antibiotic resistance in important strains such as Escherichia coli and Klebsiella pneumoniae (20,21).
AmpC Beta-lactamases are encoded by different genes such as blaCMY, blaFOX, blaDHA, blaMOX, and blaCIT. However, there is little information about the frequency of these plasmid genes and their genetic pattern in Iran.
The outbreak of antibiotic resistance is increasing, and one of the essential resistance mechanisms is the generation of extended-spectrum ESBL and AmpC by gram-negative bacilli. Therefore, determining its prevalence in each region, like hospitals, is crucial. Also, it seems that gene changes of resistant microorganisms can be different in each region, and monitoring the changes can help determine better and more appropriate treatments, especially in more sensitive units such as intensive care units and infectious diseases units. Hence, this research attempted to characterize the outbreak of fast-growing gram-negative bacilli producing ESBL and AmpC and evaluate their common genetic variants.
Bacterial Isolates
This cross-sectional research was carried out on 9424 clinical samples (including blood, urine, BAL, trachea, or Shaldon) of patients admitted to different wards of Rasool-e-Akram Hospital (Tehran, Iran) from 2019 to 2020. The samples were sent to the hospital's laboratory at the request of physicians to detect and isolate various fast-growing gram-negative bacilli. The isolates were recognized using phenotypic techni-ques.
Antibiotic Susceptibility Testing
The pattern of resistance to 8 antibiotics was identified considering Clinical and Laboratory Stand-ards Institute (CLSI) guidelines by disk diffusion method by Rosco Neosensitabs (Rosco Diagnostica, Taastrup, Denmark) (Table 1). Imipenem (10mcg), Meropenem (10mcg), Ampicillin (10mcg), Penicillin (10IU), Aztreonam (30mcg), Cefotaxime (30mcg), Cefoxitin(30mcg), and Ceftazidime (30mcg) were used in this study.
Table 1. CLSI guidelines (2020) of current antibiotics against gram-negative bacilli
| Antibiotic | S(mm or more) | I(mm) | R(mm or more) |
| Ceftazidime | 21 | 18–20 | 17 |
| Cefotaxime | 26 | 23–25 | 22 |
| Ampicillin | 17 | 14–16 | 13 |
| Penicillin | 17 | 14–16 | 13 |
| Imipenem | 23 | 20-22 | 19 |
| Meropenem | 23 | 20-22 | 19 |
| Aztreonam | 21 | 18-20 | 17 |
| Cefoxitin | 18 | 15-17 | 14 |
If the activity of the above antibiotics, except for Imipenem (meropenem) and Cefoxitin, were inhibited in the culture medium, the presence of ESBL was suspected. In this case, their existence was confirmed by confirmatory tests, which was the use of clavulanic acid (beta-lactamase inhibitor).
Screening for ESBL-producing Strains
Suspicious specimens were first cultured on Müller-Hinton agar (Condalab, Spain). Therefore, after suspecting the presence of ESBL, we used a disc containing clavulanic acid and compared the diameter of the inhibitory zone with and without inhibitors so that if the difference was more than 5 mm, ESBL was confirmed (Figure 1).
Figure 1. Evaluation of antibiotic resistance for ESBL-Producing gram-negative bacilli
Screening for AmpC-producing Strains
In addition, we were suspected of having AmpC if, in the first stage, all antibiotics, except imipenem (or meropenem), were inhibited in the culture medium, or the second stage, ESBL confirmation tests were negative. Therefore, we used cefoxitin-cloxacillin (CFO-CX) disc. A distinction between the cefoxitin-cloxacillin inhibition zone and the cefoxitin alone zone of ≥4 mm indicated AmpC production (Figure 2).

Figure 2. Evaluation of antibiotic resistance for AmpC-Producing gram-negative bacilli
Molecular Specification of ESBL and AmpC-betalactamases Genes
Finally, special primers were planned for the extracted bacteria to study the genes of blaTEM, blaSHV, and blaCTX-M in ESBL, and blaFOX, blaMOX, blaCIT, blaDHA, and blaEBC for AmpC (Table 2) (22, 23).
Table 2. Oligonucleotide sequences used in this study
| Target Gene | Primer Sequencing | Annealing Temperature | Product Size(bp) | ||
| CTX-M |
|
58 | 863 | ||
| TEM |
|
65 | 445 | ||
| SHV |
|
58 | 237 | ||
| CIT |
|
61 | 462 | ||
| FOX |
|
63 | 190 | ||
| MOX |
|
55 | 517 | ||
| EBC |
|
63 | 302 | ||
| DHA |
|
63 | 405 |
Multiplex PCR (Bio-Rad Thermal Cyclers, Singapore) was utilized to identify the major ESBL and AmpC beta-lactamase genes. Single colonies of each isolate were cultivated, and bacterial cells were bulleted by centrifugation (14000 g for 4 min) after removing supernatants. DNA extraction was carried out exploitation the microorganism GeneAll KIT (Seoul, Korea). 3 μL of Total DNA (10 ng) was added to every multiplex PCR reaction. The reaction mixture contained 12 μL mastermix (Tempase hot begin, Amplicon, Denmark), 0.7 μL specific primer (10 Pmol), and 5 μL nuclease-free water. In this study, thermal cycling PCR (BIO-RAD) was used according to the following protocol:
The primary denaturation was executed at 95°C for 15 min, followed by 29 cycles of denaturation at 94°C for 30 s, tempering at 54°C for 80 s, extension at 72°C for 1 min and final extension at 72°C for 10 min.
Finally, the Amplified genes were loaded on 2% agarose gel and electrophoresed (Gelldoc, Iran). The PCR product was stained by SYBR stain and visualized by a gel documentation system according to standard procedures.
It is necessary to explain that to carry out this project, no additional sampling was taken from the hospitalized patients. All the steps were taken on the same samples sent to the hospital laboratory at the request of the physicians.
Results of the actuarial assay were reported as mean ± standard deviation (SD) for quantitative variables and were epitomized by frequency (perce-ntage) for classified variables. Contiguous variables were evaluated using t-test or Mann-Whitney test whenever the data did not appear to have normal dispensation or when the presumption of similar variances was breached across the study groups. On the other hand, categorical variables were evaluated using the Chi-square test. A P-value of ≤ 0.05 was considered statistically significant. The statistical software SPSS version 23.0 for windows (IBM, Armonk, New York, USA) was used for the actuarial assay.
Bacterial Isolation
In the present research, Out of 9424 samples sent to the laboratory, 288 suspected cases were identified and after confirmatory tests, finally 80 ESBL and AmpC beta-lactamase productive isolates were detected. From these isolates, 75 cases are ESBL producers, and 5 cases are co-producers of ESBL and AmpC.
Because of few cases of co-producers, a total of 75 specimens were extracted and studied. In total, 31 cases (41.3%) were male with mean age 56.8±35.23 years, and 44 cases (58.7%) were female with mean age 55.21±46 years. Specimens were accumulated from various hospital wards (details in Table 3). Also, the extracted samples included urine, BAL, blood, trachea, and Shaldon secretions that the details are described in Table 3.
Table 3. Characteristics of the studied samples
| Character | Category | Number (percent) |
| Gender | Man | 31 (41.3) |
| Female | 44 (58.7) | |
| Inpatient department |
Emergency | 15 (20.0) |
| Respiratory disease | 15 (20.0) | |
| Internal medicine | 9 (12.0) | |
| Infectious disease | 6 (8.0) | |
| Respiratory ICU | 7 (9.3) | |
| MICU | 5 (6.7) | |
| Gynecology | 4 (5.3) | |
| Surgery | 4 (5.3) | |
| Pediatrics | 3 (4.0) | |
| post CCU | 2 (2.7) | |
| Surgical ICU | 1 (1.3) | |
| Open heart ICU | 1 (1.3) | |
| Ophthalmology | 1 (1.3) | |
| Skin disease | 1 (1.3) | |
| Sample type | Urine | 57 (76.0) |
| Trachea | 1 (1.3) | |
| Blood | 4 (5.3) | |
| BAL | 12 (16.0) | |
| Shaldon | 1 (1.3) | |
| Mean age, year | 55.43±20.36 | |
The types of bacilli identified were E. coli in 44 cases (58.7%), K. pneumoniae in 25 samples (33.3%), Pseudomonas aeruginosa in 3 samples (4.0%), Enter-obacter cloacae in 2 samples (2.2%) and Acinetobacter baumannii in 1 sample (1.3%). The above study and the information obtained were the results of 75 ESBL producers. The frequency of gram-negative bacilli in terms of primary characteristics is listed in detail in Table 4.
Table 4. Distribution of ESBL gram-negative bacilli according to study characteristics
| Bacillus | E. coli | Klebsiella | Pseudomonas aeruginosa | Enterobacter cloacae | Acinetobacter baumannii | P-value |
| Gender | 0.270 | |||||
| Man | 54.8% | 32.3% | 9.7% | 3.2% | 0.0% | |
| Female | 61.4% | 34.1% | 0.0% | 2.3% | 2.3% | |
| Mean age, year | 54±20 | 57±18 | 56±30 | 72±11 | 60±10 | 0.779 |
| Inpatient department | 0.001 | |||||
| Emergency | 80.0% | 13.3% | 6.7% | 0.0% | 0.0% | |
| Respiratory disease | 53.3% | 33.3% | 6.7% | 6.7% | 0.0% | |
| Internal medicine | 55.6% | 33.3% | 11.1% | 0.0% | 0.0% | |
| Infectious disease | 66.7% | 33.3% | 0.0% | 0.0% | 0.0% | |
| Respiratory ICU | 14.3% | 71.4% | 0.0% | 0.0% | 14.3% | |
| MICU | 20.0% | 80.0% | 0.0% | 0.0% | 0.0% | |
| Gynecology | 75.0% | 25.0% | 0.0% | 0.0% | 0.0% | |
| Surgery | 100% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Pediatrics | 100% | 0.0% | 0.0% | 0.0% | 0.0% | |
| post CCU | 50.0% | 50.0% | 0.0% | 0.0% | 0.0% | |
| Surgical ICU | 100% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Open heart ICU | 100% | 0.0% | 0.0% | 0.0% | ||
| Ophthalmology | 100% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Skin disease | 0.0% | 100% | 0.0% | 0.0% | 0.0% | |
| Sample type | 0.001 | |||||
| Urine | 73.7% | 22.8% | 1.8 | 0.0% | 1.8% | |
| Trachea | 100% | 0.0% | 0.0% | 0.0% | 0.0% | |
| Blood | 0.0% | 50.0% | 0.0% | 50.0% | 0.0% | |
| BAL | 8.3% | 75.0% | 16.7% | 0.0% | 0.0% | |
| Shaldon | 0.0% | 100% | 0.0% | 0.0% | 0.0% |
Antibiotic Susceptibility Results
First, there was no difference in the frequency of bacterial strains between men and women based on different age groups. However, isolated Bacillus strains have a great variety in other hospital wards, which are described in detail in Table 4, with the names of each ward. Of course, as detailed in Table 4, the isolated strains also varied according to the type of samples. For example, in urine, the most common isolated strain was E. coli (73.7%), and there were no Enterobacter cloacae, while in blood, E. cloacae (50%) and Klebsiella pneumoniae (50%) were the most common. Regarding antibiotic resistance against ESBL-producing gram-negative bacilli, the frequency of antibiotic resistance in detail is expressed in Table 5. For example, the highest antibiotic resistance against gentamycin is seen in Enterobacter cloacae (100%) and Acinetobacter baumannii (100%), while Pseudomonas aeruginosa had no resistance to gentamycin.
Table 5. Distribution of antibiotic resistance by type of gram-negative bacilli
| Antibiotic | E. coli | Klebsiella | Pseudomonas aeruginosa | Enterobacter cloacae | Acinetobacter baumannii | P-value |
| Gentamycin | 34.7% | 76.0% | 0.0% | 100% | 100% | 0.002 |
| Cefepime | 77.6% | 44.0% | 33.3% | 0.0% | 100% | 0.009 |
| Imipenem | 8.2% | 56.0% | 66.7% | 0.0% | 100% | 0.001 |
| Ampicillin-Sulbactam | 34.7% | 84.0% | 66.7% | 50.0% | 0.002 | |
| Nitrofurantoin | 8.2% | 16.0% | 0.0% | 0.0% | 100% | 0.049 |
| Amikacin | 6.1% | 60.0% | 66.7% | 0.0% | 100% | 0.001 |
| Cefoxitin | 0.0% | 0.0% | 33.3% | 0.0% | 0.001 | |
| Ciprofloxacin | 75.0% | 88.0% | 66.7% | 0.0% | 100% | 0.050 |
| Cefazolin | 95.9% | 52.0% | 33.3% | 0.0% | 100% | 0.001 |
| Cefotaxime | 100% | 100% | 66.7% | 100% | 100% | 0.001 |
| Trimethoprim-sulfamethoxazole | 83.7% | 88.0% | 100% | 100% | 100% | 0.862 |
| Piperacillin-tazobactam | 4.1% | 48.0% | 66.7% | 66.7% | 66.7% | 0.001 |
| Ceftriaxone | 50.0% | 0.0% | 0.001 | |||
| ceftazidime | 4.1% | 48.0% | 33.3% | 0.0% | 0.0% | 0.001 |
| Meropenem | 44.0% | 66.7% | 0.0% | 0.0% | 0.001 | |
| Cefuroxime | 2.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.958 |
The total frequencies of common genes of ESBL-producing gram-negative bacilli were blaCTX-M in 76.0%, blaTEM in 46.7%, and blaSHV in 26.7%. While in 14.7%, the type of gene detected was unknown. Also, cases of simultaneous detection of multiple genes were observed in 49.3% of gram-negative ESBL-producing bacilli; of this number, triple gene detection was reviewed in 16.0% (Figures 3, 4, 5, and 6).
In this article, we used positive control to identify blaSHV, blaCTX-M, and blaTEM genes. Still, for blaDHA, blaMOX, blaCIT, blaEBC, and blaFOX genes, positive control species could not be found because no study was done previously. The frequency of genes by sex, age, type of bacilli, and sample evaluated are described in Table 6.
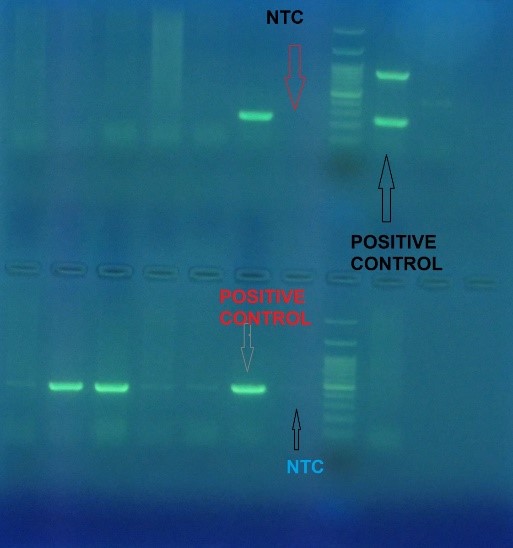
Figure 3. PCR Multiplex for detection of TEM (lower part) & SHV(upper part) gene
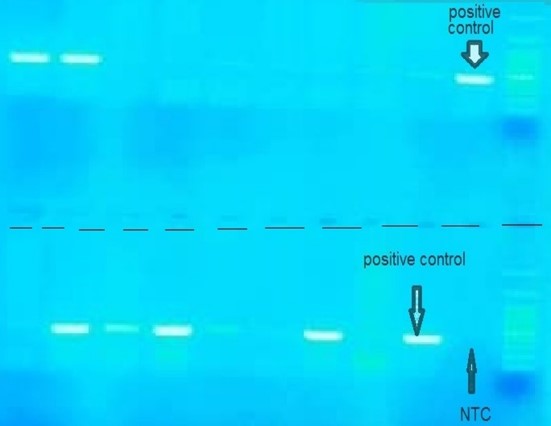
Figure 4. PCR Multiplex for detection of CTX-M gene
.jpg)
Figure 5. PCR Multiplex for detection of CIT&EBC&-DHA&FOX gene
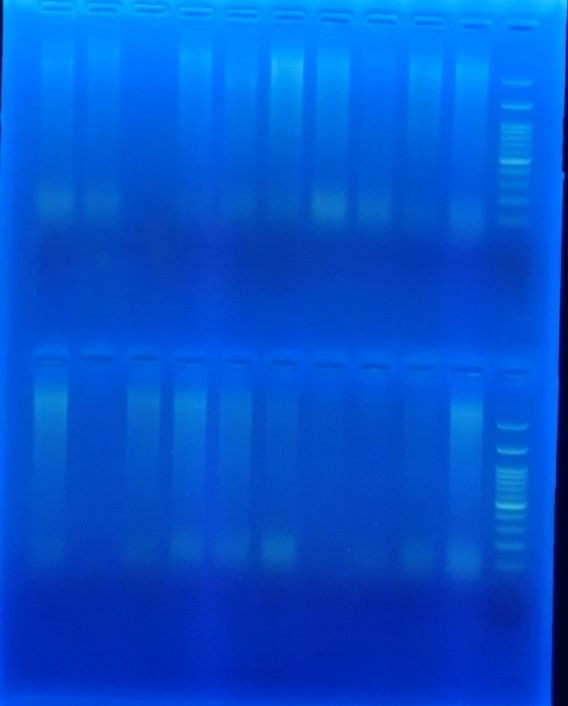
Figure 6. PCR Multiplex for detection of MOX gene
Table 6. Frequency of common genes of ESBL gram-negative bacilli according to study characteristics
| GENE | CTX-M | TEM | SHV | unknown | Multi |
| Total | 76.0% | 46.7% | 26.7% | 14.7% | 49.3% |
| Gender | |||||
| Man | 64.5% | 48.4% | 22.6% | 25.8% | 47.4% |
| Female | 84.1% | 45.5% | 29.5% | 6.8% | 50.0% |
| Mean age, year | 56.56 | 54.97 | 58.86 | 55.40 | 55.39 |
| Sample type | |||||
| Urine | 77.2% | 50.9% | 17.5% | 14.0% | 47.4% |
| Trachea | 0.0% | 100% | 0.0% | 0.0% | 0.0% |
| Blood | 100% | 0.0% | 0.0% | 0.0% | 0.0% |
| BAL | 66.7% | 33.3% | 58.3% | 25.0% | 58.3% |
| Shaldon | 100% | 100% | 100% | 0.0% | 100% |
| Type of bacilli | |||||
| E. coli | 77.3% | 45.5% | 9.1% | 13.6% | 43.2% |
| Klebsiella | 72.0% | 52.0% | 60.0% | 20.0% | 64.0% |
| Pseudomonas aeruginosa | 66.7% | 33.3% | 33.3% | 0.0% | 33.3% |
| Enterobacter cloacae | 100% | 0.0% | 0.0% | 0.0% | 0.0% |
| Acinetobacter baumannii | 100% | 100% | 0.0% | 0.0% | 100% |
The relationship between gram-negative bacilli's common genes and antibiotic resistance is illustrated in Table 7.
Table 7. Frequency of antibiotic resistance according to common genes of gram-negative bacilli
| Antibiotic | CTX-M | TEM | SHV | Unknown | Multi |
| Gentamycin | 45.9% | 45.0% | 61.9% | 20.0% | 45.2% |
| Cefepime | 68.9% | 60.0% | 61.9% | 40.0% | 66.7% |
| Imipenem | 23.0% | 27.5% | 42.9% | 0.0% | 23.8% |
| Ampicillin-Sulbactam | 47.5% | 42.5% | 61.9% | 0.0% | 42.9% |
| Nitrofurantoin | 9.8% | 12.5% | 9.5% | 0.0% | 9.5% |
| Amikacin | 23.0% | 35.0% | 42.9% | 20.0% | 28.6% |
| Cefoxitin | 1.6% | 2.5% | 0.0% | 0.0% | 2.4% |
| Ciprofloxacin | 75.4% | 71.8% | 81.0% | 100% | 75.6% |
| Cefazolin | 82.0% | 80.0% | 66.7% | 100% | 83.3% |
| Cefotaxime | 98.4% | 67.5% | 100% | 100% | 97.6% |
| Trimethoprim-sulfamethoxazole | 88.5% | 92.5% | 85.7% | 100% | 92.9% |
| Piperacillin-tazobactam | 16.4% | 20.0% | 33.3% | 0.0% | 16.7% |
| Ceftriaxone | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| Ceftazidime | 16.4% | 17.5% | 33.3% | 0.0% | 0.0% |
| Meropenem | 13.1% | 15.0% | 28.6% | 0.0% | 14.3% |
| Cefuroxime | 1.6% | 0.0% | 0.0% | 0.0% | 0.0% |
A large group of genes associated with ESBL and AmpC beta-lactamases expression is involved in inducing antibiotic resistance in various bacteria. Identifying these genes and assessing their detection will be crucial in determining the pattern of antibiotic resistance. In other words, a significant proof for the change in the drug resistance of bacteria (intensification or reduction of resistance) is the change and occurrence of mutations in these genes and the change in their expression. Therefore, detection of these genes can be important at the clinical level and facilitate the traceability of changes in antibiotic resistance in different parts of the hospital. The current research identified the frequency of fast-growing gram-negative bacilli producing ESBL and studied their common genetic changes in Rasool-e-Akram Hospital. In this evalu-ation, ESBL-producing gram-negative Bacilli strains were first identified in various blood, urine, trachea, Shaldon, and BAL samples by cultivating in the mentioned methods.
Remarkable results in the present study were:
A) The most common cultivated strains were E. coli (58.7%), K. pneumoniae (33.3%), P. aeruginosa (4.0%), Enterobacter cloacae (2.7%), and Acinetobacter baumannii (1.3%);
B) Regarding antibiotic resistance evaluated in different sections, the highest antibiotic resistance was related to cefotaxime with a resistance of 98.8%, trimethoprim-sulfamethoxazole with 86.2%, cefazolin with 77.5%, ciprofloxacin with 76.0%, and cefepime with the resistance of 63.8%. In this regard, the lowest level of resistance was related to colistin with no identified resistance, cefoxitin and ceftriaxone with 1.2% resistance;
C) The communication between detecting some bacterial genes and antibiotic resistance was notable. First, the level of drug resistance to imipenem in people who harbored the blaSHV gene was significantly higher than in the cases that did not harbor it. Also, similarly, drug resistance to amikacin and ceftazidime was significantly higher in those who detected the blaSHV gene. Therefore, studying the role of the blaSHV bacterial gene in increasing antibiotic resistance to drugs such as imipenem, amikacin, and ceftazidime, which are widely used drugs in various sectors, suggests that the blaSHV bacterial gene evaluation can be routinely used to evaluate the antibiotic resistance pattern of ESBL-producing gram-negative bacilli.
Based on the different characteristics of different communities and the genomic pattern of bacteria in other communities, a different expression of bacterial genes was obtained simultaneously with antibiotic resistance, which could be specific to that community. In the study by Gundran et al. (24), the highest prevalence was related to the blaCTX-M-1 gene, with a prevalence of 72.4%, followed by the blaCTX-M-2 gene. The blaTEM and blaSHV genes frequency were 57.9% and 27.5%, respectively. Simultaneous expression of blaCTX-M was observed in 73.5% of patients, utterly different from the values obtained in the existing research. In the study by Pishtiwan et al. (25), of the ESBL-producing E. coli cases, 81% displayed the blaTEM gene, 16.2% the blaSHV gene, and 32.4% the blaCTX-M gene. Similarly, 64.7% showed blaTEM gene, 35.2% blaSHV gene, and 41.1% blaCTX-M gene in K. pneumoniae samples. In the study of Farzi et al. (26), from 100 urine samples, 21 ESBL producer cases (21%) were detected, and the presence of blaCTX-M and blaTEM genes in the isolates were 21% and 20%, respectively. In the study of Roshdi Maleki et al. (27), of 120 strains of K. pneumoniae, 71 (59.2%) were established for ESBL. The blaTEM and blaSHV ESBLs were identified in 35 (49.3%) and 31 (43.7%) strains respectively. finally, the co-existence of blaTEM and blaSHV was detected in 5 (7%) isolates. In a study by Bajpai et al. (28) in India, genes related to beta-lactamase production were examined, including blaCTX-M-M (48.7%), blaTEM (7.6%), and blaSHV (1.5%). In a study by Ghaima et al. (29) that investigated ESBL genes in Acinetobacter baumannii isolates, blaCTX-M-M, blaTEM, and blaSHV genes were discovered in 45%, 75%, and 27.5%, respectively. In a study by Kumarss Amini et al. (30) in Iran, from 200 diarrhea specimens, 60 (30%) Shigella sonnei strains were found. The molecular test results showed that 40 (66.6%) and 33 (55%) of the strains harbored the blaCTX-M-8 and CMY genes, respectively.
The blaCTX-M-2 gene was not identified in any of the specimens. Also, in the study of Mubarak Saif Alfaresi et al. (31) in the UAE, out of 240 cases of ESBL, 228 cases carried the ESBL bla gene. Also, 87% of the strains carried the blaCTX-M-15 gene, and the blaSHV-28 gene was identified in 13% of these strains. Kooshesh et al. (32) found that in ESBL and AmpC positive isolates in Kerman in 2015, 76.1% were positive for blaTEM, 14.2% positive for blaOXA, and 2.3% positive for blaSHV, and none of the isolates were found positive for blaPER. The outbreak of ESBL, AmpC, blaTEM, and blaOXA in inpatients isolates was 7.2%, 0.2%, 37.2%, and 8.5, respectively, which differed from our study terms of genotype and destination.
As it is evident, in different societies and even in a society with different geographical conditions, both the rate of antibiotic resistance and the expression of bacterial genes associated with these resistances have been reported quite differently, which can be related to both the genomic conditions associated with that community and differences in the techniques used to assess genomic expression.
Our findings showed that antitoxin A antibodies can play a greater protective effect than P. aeruginosa infections. This conjugated compound increases the level of anti-ALG IgG antibodies, and immunization with this type of conjugate can stimulate toxin A-neutralizing antibodies. T
None.
None.
Study Conception, Design, Data Analysis and Interpretation of Results: Dr. Pegah Babaheidarian, Majid Mehdinejad. Design and Interpretation of Results: Dr. Ali Zare-Mirzaie. Data Collection: Roya Mokarinejad, Ensieh Jafari.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2021/09/7 | Accepted: 2022/01/15 | ePublished: 2022/02/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









