BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1472-en.html

 , Mohsen Arzanlou1
, Mohsen Arzanlou1 

 , Amir Teimourpour2
, Amir Teimourpour2 

 , Majid Esmaelizad3
, Majid Esmaelizad3 

 , Mehdi Yousefipour4
, Mehdi Yousefipour4 

 , Jafar MohammadShahi5
, Jafar MohammadShahi5 

 , Roghayeh Teimourpour6
, Roghayeh Teimourpour6 


2- Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
3- Central Labaratory, Razi Vaccine and Serum Research Institute, Karaj, Iran
4- Department of Infectious and Tropical Diseases, Tehran University of Medical Sciences, Tehran, Iran
5- Departments of Infectious Diseases, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran
6- Department of Microbiology, School of Medicine, Ardabil University of Medical Sciences, Ardabil Iran ,
Escherichia coli is a member of the Enterobac-teriaceae family. The intestinal tract of humans and warm-blooded animals is its natural habitat. Also, its presence in water and food indicates contamination with the feces (1). Even though E. coli is one of the most prevalent commensal, it has five pathotypes that are associated with intestinal and extraintestinal disease, including enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), entero-aggregative E. coli (EAEC), diffusely-adherent E. coli (DAEC), adherent-invasive E. coli (AIEC) and uropa-thogenic E. coli (UPEC) (1). These pathotypes differ in the type of disease and related virulence factors such as adhesions, toxins, polysaccharide capsules, invasins, extragenetic material, and pathogenicity-associated islands (PAIs). UPEC is typically responsible for over 90% of urinary tract infections (UTI). UTI is defined as inflammation and disorder of the urinary tract due to the invasion of pathogens. Clinical features of the disease range from urethritis and cystitis to severe complications like kidney infection, pyelonephritis, and even sepsis. According to previous studies, UTI is the most prevalent infectious disease worldwide. The prevalence of this disease in women is higher than in other people. It is estimated that approximately 40% to 60% of women encounter UTI at least once in their lifetime (2). Integrons are mobile genetic elements that can be found in plasmids, transposons, and chromosomes. They were primarily discovered in antibiotic resistance research. They had an essential role in acquiring, expressing, and disse-minating resistant genes among pathogens, especially gram-negative bacteria, through horizontal gene transfer (HGT) movement. The structure of integrons principally consists of a gene expressing recombinases or integrase enzyme (intI gene), which is mediated in gene cassettes insertion, recombination site (attI), which is identified by the integrase, and a promoter (Pc) that facilitates transcription of gene cassettes. Thus far, four classes of integrons based on intI gene similarity and about 200 gene cassettes conferring resistance to aminoglycoside, β-lactams, chloram-phenicol, quinolones, and trimethoprim have been detected. Incorporating more than one cassette into the integron is associated with resistance to several antibiotics and multiple drug resistance (MDR) strains. Previous studies confirmed a strong relationship between the presence of integrons and multiple drug resistance, especially in Enterobacteriaceae family members, which are also known as very opportunistic agents (3-5).
Extended-spectrum beta-lactamases (ESBLs) are a group of beta-lactamase enzymes that inactivate powerful antibiotics such as third-generation of cephalosporins. However, these bacteria are suscep-tible to cephamycins and carbapenems, as well as to the beta-lactamase-inhibitor compound. Although ESBL-producing bacteria are inhibited by cefamycins, AmpCtype β-lactamase-producing bacteria can inactivate these agents. The genes expressing AmpC type β-lactamases can be determined on a chrom-osome or plasmid. In gram-negative bacteria, it is usually chromosomal and inducible. Despite ESBLs, AmpC type β-lactamases can also inactivate the beta-lactam/beta-lactam inhibitor combination. Plasmid-mediated AmpC lactamases are a severe threat in hospitals because these plasmids are easily trans-mitted through horizontal gene transfer way among species and mediated in nosocomial outbreaks.
Therefore, it is essential to identify molecular mechanisms by which bacteria can spread resistance genes among themselves and identify indigenous strain antibiotic resistance patterns. These data will help increase our awareness of the antibiotic resistance mechanisms and provide the best empirical therapy. In this regard, this work aimed to study the antibiotic susceptibility pattern of UPEC, prevalence of the classes 1, 2, 3 integrons, and prevalence of ESBLs and AmpC producing strains.
In this descriptive, cross-sectional study, a total of 163 E. coli isolates were recovered from urine specimens of patients admitted to Bu Ali and Imam Khomeini teaching Hospitals of Ardabil University of medical science in Iran (2017-2018). After obtaining written informed consent (ethical code: IR.ARUMS.REC. 1396.69), each patient's demographic data, including age, sex, and underlying disease, were recorded. Biochemical tests, such as gram staining, oxidase, and catalase tests, indole production, methyl red, Voges-Proskauer (VP), Simmons citrate, hydrogen sulfide, and urea hydrolysis tests were employed for primary identification and phenotypic detection of E coli isolates (6). The polymerase chain reaction (PCR) method was used for confirming the results acquired from the phenotypic tests by targeting 16srRNA specific gene as described previously (7). All isolates were stored at −80°C in a freezing solution containing trypticase soy broth (Padtan Teb Co., Iran) and 20% glycerol (6).
Antimicrobial Susceptibility Testing
Kirby-Bauer disk diffusion method was employed to determine the antimicrobial susceptibility profile of isolates to 12 antibiotics based on the Clinical and Laboratory Standards Institute (CLSI) guidelines (8). The antibiotic discs were Ampicillin 10 μg, Cefazolin 30μg, Cefotaxime 30 μg, Ceftazidime 30 μg, Cefepime 30 μg, Ceftriaxone 30 μg, Imipenem 10μg, Gentamicin 10μg, Amikacin 30μg, Nitrofurantoin 300 μg, Ciprofloxacin 5 μg and Trimethoprim- sulfamethox-azole 1.25/23.75 μg (9). In this study, E. coli ATCC 25922 strain was used as a control for drug susceptibility testing. Isolates were resistant to at least one antibiotic in three or more antibiotic classes were considered as MDR strain.DNA Extraction
DNA was extracted from colonies of E. coli isolates using the boiling method (10). In brief, the colonies were picked up from 18 h fresh culture nutrient agar plates and dissolved in 200 μL of DNA extraction buffer (Tris-Hcl100 mM, pH=7.6, Tween20 0.05%, proteinase K 200 μg/mL), incubated at 55°C for 3 h followed by 100°C for 10 min. After centrifugation at 5000 × rpm for 10 min, the supernatant was used as a template for PCR.
Detection of Class 1, 2 and 3 Integrons by Polymerase Chain Reaction (PCR)
For detection of integrons in MDR E. coli isolates, 3 specific sets of primers were used as described in the study by Kargar et al. (11)(Table1). All primers were provided by Cinnagene Co. (Tehran, Iran). The PCR reaction was prepared in a total volume of 25 µL containing 1xPCR buffer, 10 mM dNTP mix, 1.5 mmol MgCl2, 0.2 mM of each dNTP, 1U of Taq DNA polymerase, 5-10 pmol of each primer, 0.5-1 µg of template DNA. The temperature profile for each gene is presented in Table 1. Finally, the PCR products were sequenced on one strand by Pishgam Biotech co. (Tehran, Iran). Obtain results were analyzed by DNAMAN and BLAST soft wares and submitted in Genbank (accession numbers: MK994976.1, MK994-977.1). A PCR reaction without DNA or primers was used as a negative control.Phenotypic Evaluation of Broad-Spectrum β-lactamase Production
Determination of ESBL-producing strains was performed based on CLSI protocols using Double Disc Diffusion Test (DDDT) (12). The bacterial suspension (concentration of suspension adjusted to McFarland standard 0.5) cultured on Müller Hinton medium. Cefotaxime (30 μg) and cefotaxime (30 μg) / clavulanic acid (10 μg) disk (padtanteb, Iran) were placed on the medium at a distance of 2 cm from each other. After 24 hours of incubation at 37°C, the growth inhibition zone was examined. In strains with a growth inhibition zone of 5 mm or more around the cefotaxime / clavulanic acid disk compared to the growth inhibition zone around the cefotaxime disk, it was considered the ESBL-producing strain. After identifying the strains producing ESBLs enzymes, the prevalence of CTX-M, SHV, TEM genes was determined using Table 1 universal primer. Standard strain E. coli 25922 ATCC was used as the negative control of the test.AmpC Screening using the Disk Diffusion Method
AmpC beta-lactamase-producing strains were identified using a cefoxitin screening test. In this test, a cefoxitin disc (cefoxitin30g) was used to screen AmpC beta-lactamase-producing strains on the strains that were not identified as producing ESBL enzymes. For this purpose, after culturing the bacterial suspension following the 0.5 McFarland standard, cefoxitin was placed on plates containing Müller-Hinton agar medium. The diameter of the zone of inhibition less than or equal to 18 mm indicated the presence of AmpC inducible beta-lactamase (13).Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction (ERIC-PCR)
The ERIC-PCR was done in a total volume of 20 μL, containing 1x PCR buffer, 0.1% tween20, dNTPs 250 µM, 20 pmol of each primer, Mgcl2 3.75 mM, Taq DNA polymerase 2units, DNA template 100 ng. Expected amplified products were separated by electrophoresis on 1.5% agarose gel along with 100 bp DNA marker (Cinnaclone, Iran) stained with a green viewer (Pars Tous, Iran) and visualized under UV illumination (VilbertLourmat Co., Japan) (14).In order to cluster the isolates, based on band scoring, a matrix with 38 rows and 20 columns was prepared in which zero indicates the absence and one indicates the expression of the target gene in each isolate. The dissimilarity matrix and dendrogram plot were constructed using the 'binery' method and ward D method, respectively. All statistical calculations were performed using R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Statistical Analysis
Statistical analyses were done by SPSS version 22 (SPSS Inc., Chicago, IL., USA). The Chi-square and Mann–Whitney tests were employed to estimate the relationship between variables. The significance level was considered less than 0.05 (P<0.05).
Of the 163 studied patients, 107 cases were female (66%), and 56 were male (34.4%). The average age of the patients was 31.96 ±30.6, with a mean of 20 years and a range of one year to 94 years. The ratio of women to men was 1:91 (P<0.001), which indicated a significant relationship between the age of men and women. Also, 84 cases (51.5%) were from Imam Khomeini Hospital, and 79 cases (48.5%) from Bou Ali Hospital, and 138 (84.7%) cases were multiple drug resistance strains (MDR). Biochemical tests along with amplification of the specific region of 16srRNA were employed for the identification of UPEC strain. The amplification of 919 bp fragment of 16srRNA of UPEC is presented in Figure 1.
Table 1. Sequences of Primers Used for identification of intI-1, intI-2 and intI-3, 16srRNA and molecular typing
| Reference | PCR conditions | PCR product size (bp) | Primer sequence (5'-3') | Target region |
| (14) | 7min at 95°C; 35 cycles of 30 sec at 90°C, 1 min at 52°C, 3 min at 65°C; 16 min at 65°C | variable | ATG TAA GCT CCT GGG GAT TCA C | ERIC 1 |
| AAG TAA GTG ACT GGG GTG AGC G | ERIC 2 |
|||
| (11) | 5min at 95°C; 35 cycles of 1 min at 95°C, 1 min at 60°C, 2 min at 72°C; 5 min at 72°C | 436 bp | GGT CAA GGA TCT GGA TTT CG | Int1-F |
| ACA TGC GTG TAA ATC ATC GTC | Int1-R | |||
| (11) | 5 min at 95°C; 35 cycles of 1 min at 95°C, 1 min at 60°C, 1 min at 72°C; 5 min at 72°C | 788 bp | CAC GGA TAT GCG ACA AAA AGG T | IntI2-F |
| GTA GCA AAC GAG TGA CGA AAT G | IntI2-R | |||
| (11) | 5 min at 95°C; 35 cycles of 1 min at 95°C, 1 min at 60°C, 1 min at 72°C; 5 min at 72°C | 600 bp | AGT GGG TGG CGA ATG AGT G | IntI3-F |
| TGT TCT TGT ATC GGC AGG TG | IntI3-R | |||
| (7) | 919 | F- AGAGTTTGATCMTGGCTCAG | 16srRNA-F | |
| R- CCGTCAATTCATTTGAGTTT | 16srRNA-R | |||
| (15) | 5 min at 94°C; 32cycles of 45sec at 94°C, 42sec at 50°C, 1 min at 72°C; 5 min at 72°C | 1018 | ATT TGT CGC TTC TTT ACT CGC | SHV-F |
| TTT ATG GCG TTA CCT TTG ACC | SHV-R | |||
| (15) | 5 min at 94°C; 32cycles of 45sec at 94°C, 42sec at 50°C, 1 min at 72°C; 5 min at 72°C | 544 | ATG TGC AGY ACC AGT AAR GT | CTX-M-1 |
| TGG GTR AAR TAR GTS ACC AGA | CTX-M-2 | |||
| (15) | 10 min at 94°C; 32cycles of 1 min at 94°C, 1 min at 56°C, 1 min at 72°C; 5 min at 72°C | 1076 | ATA AAA TTC TTG AAG ACG AAA | TEM-F |
| GAC AGT TAC CAA TGC TTA ATC | TEM-R |
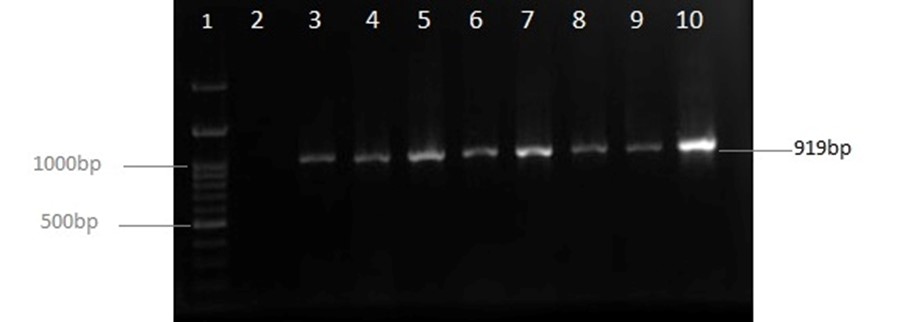
Figure 1. Illustration of 16SrRNA PCR product on 1% Agarose gel; lane 1: Size Marker (Ladder100bp: CinnaClon, Iran); lane 2: Negative control; lane 4 to 9: Samples.
The highest and the lowest antibiotic resistance was against ampicillin (89.6%) and nitrofurantoin (1.2%), respectively. Resistance to other antibiotics was as follows: cefazolin 60.7%, cefepime 42.3%, cefotaxime 53.4%, ceftriaxone 49.1%, ceftazidime 49.7%, imipe-nem 36.2%, gentamicin 20.2%, amikacin 6.7%, ciprof-loxacin 41.1% and sulfamethoxazole-trimethoprim 73.6% (Table 2).
Statistical analysis indicated a significant relationship between increasing age and increasing drug resistance (P<0.001). Also, a significant relati-onship was observed between sex and cotrimoxazole, cefotaxime, ciprofloxacin, and ceftriaxone antibiotics. The resistance rate of ceftriaxone (P=0.023), cefotaxime (P=0.049), and ciprofloxacin (P=0.033) were higher in men than in women. Still, about sulfamethoxazole-trimethoprim antibiotic, the resist-ance rate in women was higher than in men (P<0.001). A significant relationship was reported between the ciprofloxacin, gentamicin, nitrofurantoin, cefotaxime, ceftriaxone antibiotics with diabetes. The Resistance to nitrofurantoin (P=0.003), ceftriaxone (P=0.016), cefotaxime (P=0.047), imipenem (P<0.001), genta-micin (P=0.002), and ciprofloxacin (P<0.001) was signi-fcantly higher in diabetic patients compared to non-diabetic patients.
Table 2. Antibiotic susceptibility pattern results of isolates examined by disk agar diffusion method
| Resistance | Intermediate | Sensitive | ||||
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||||
| 146(89.6) | 2(1.2) | 15(9.2) | Ampicillin | |||
| 99(60.7) | 0(0) | 64(39.3) | Cefazolin | |||
| 69(42.3) | 7(4.3) | 87(53.3) | Cefepime | |||
| 4(49.1) | 80(2.5) | 79(48.5) | Ceftrixone | |||
| 87(53.4) | 1(0.6) | 75(46.0) | Cefotaxime | |||
| 81(49.7) | 10(6.1) | 72(44.2) | Ceftazidime | |||
| 59(36.2) | 6(3.7) | 98(60.1) | Imipenem | |||
| 33(20.2) | 9(5.5) | 121(74.2) | Gentamicin | |||
| 11(6.7) | 23(14.1) | 129(79.1) | Amikacin | |||
| 67(41.1) | 4(2.5) | 92(56.4) | Ciprofloxacin | |||
| 120(73.6) | 1(0.6) | 42(25.8) | Trimethoprim- Sulfamethoxazole | |||
| 3(1.2) | 2(1.8) | 158(96.9) | Nitrofurantoin | |||
The results of the PCR assay showed that the prevalence of class I and 2 integrons was 39.9% (n=65) and 14.1% (n=23), respectively. No class 3 integron was verified in any samples (Figure 2). PCR products of IntI and IntI2 were subjected to DNA sequencing by Applied Biosystems 3500 (Pishgam co., Tehran, Iran). The results of DNA sequencing were analyzed using DNAMAN v.4.0 (Lynnon Biosoft, USA) and BLAST soft wares and submitted in Genbank under accession number (MK994977.1, MK994976.1). IntI had 100% identity with other sequences in Genbank, while IntI2 had 99% identity.

Figure 2. PCR product electrophoresis for the Study of IntI and IntI2 genes on 1% agarose gel; Lane 1: DNA marker (Ladder100bp: Cinnaclon); Lane 2: Negative Control; lane 3 - 9: IntI1 (436bp) and IntI2 (788bp)
A significant association was observed between MDR strain and the presence of integrons (P=0.001, OR = 6.34; 95% CI: 1.81, 22.17), such that the presence rate of integrons in MDR positive samples was 6.34 times more than the MDR negative samples.
Statistical analyses revealed a significant relevance between the Int2 variable and the type of hospital (P=0.011). Accordingly, at Imam Khomeini Hospital, 20.2% of the samples were carriers of type 2 integron (IntI2), while at Bu Ali Hospital, this rate was 6.3%. Similar results were not seen about IntI1. Also, a statistically significant correlation between age –integron2 (P=0.008) and sex–integron1 (0.014) was observed. Out of 163 E. coli samples examined based on the phenotypic test, 76 (46.6%) samples produced ESBLs. Among the ESBLs negative strains (87 samples), 10 isolates (11.49%) were positive for AmpC prod-uction. The results of the prevalence of genes encod-ing beta-lactamase enzymes CTX-M, SHV, and TEM using PCR were 16.0, 1.8, and 14.1%, respectively.
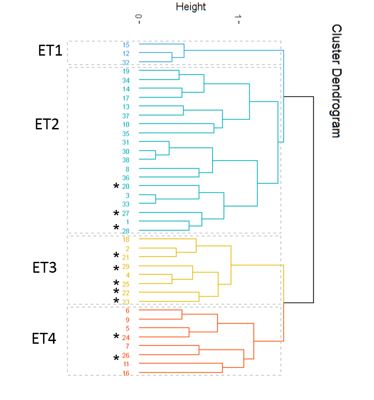
In this study, the ERIC-PCR fingerprinting method was used for differentiating and genotyping the limited number of isolates. Based on ERIC-PCR fingerprinting, 95% confidence level, and 75% similarity index, isolates were grouped into 4 clusters (ERIC types 1–4). As seen in Figure 3, most of the isolates in ET3 were taken from Bu Ali hospital, while most isolates obtained from Imam Khomeini Hospital are located in ET 2.
Figure 3. Dendrogram created from enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR) banding pattern of 38 UPEC isolated from patients admitted to teaching hospital in Ardabil province. Marked numbers were related to isolates obtained from Bu Ali hospital while rest of them were recovered from Imam Khomeini Hospital.
UTI is one of the most common bacterial infections that affects approximately 150 million people worldwide each year (16). According to a study in Iran, the prevalence of this infection is about 13.3%, and UPEC is the most common causative agent (17).
Male infants, older men, and women of all ages are prone to UTI (18). Frequent recurrences of infection, pyelonephritis associated with sepsis, and severe renal destruction are urinary tract infection complications (19). Female gender, previous history of urinary tract infections, sexual activity, diabetes, obesity, genetic susceptibility, prolonged use of the urinary catheter, and old age are the most critical risk factors for urinary tract infection(20).
Trimethoprim/Sulfamethoxazole is a good choice for treating uncomplicated UTI, but resistance rates are high, and other antimicrobial agents such as first-generation cephalosporins and fluoroquinolone should be heeded (2). MDR is defined as the resistance to at least one antibiotic in 3 or more antimicrobial classes (21).
In this study, which was performed on 163 E. coli isolates from patients with urinary tract infections, the prevalence of MDR samples was 84.7%, indicating a high level of antibiotic resistance in these isolates. The prevalence of multidrug-resistant UPEC in other sites of Iran ranges from 10.5% to 79.2% related to Kashan and Hamedan provinces, respectively (22). Also, the global prevalence of MDR strains is increasing, especially in developing countries, due to misuse and overuse of antibiotics (23, 24).
In this study, the highest resistance was reported to ampicillin (89.6%), trimethoprim-sulfamethoxazole (73.6%), and cefazolin (60.7%), while the lowest resistance was to nitrofurantoin (1.2%). The rate of imipenem antibiotic resistance was 36.2%, which was higher than in previous studies (25-27). Our study showed that nitrofurantoin, amikacin, and gentamicin are the most effective antibiotics against MDR isolates.
In a similar study, Mihankhah et al. indicated that E. coli is the most prevalent agent of urinary tract infection in Iran and the highest rate of antibiotic resistance was against ampicillin (89.29%).
In a systematic review, Alizade concluded that the prevalence of antibiotic resistance in gram-negative bacteria, especially E. coli, varies from region to region in Iran and is dramatically rising (28).
Integrons, as a mobile genetic element, have a critical role in maintaining and spreading antimicrobial resistance among bacteria, particularly gram-negative ones. It is accepted that the presence of integrons in MDR strain is substantially higher than susceptible strains (29).
Previous studies in Iran have reported that pooled prevalence rates of class 1, 2 integron are 41.7% and 17.4%, respectively. In the case of E. coli, no cases of class 3 integron have been reported except for a study conducted by Kargar et al. (11) in which the presence of class 3 integron in E. coli was reported in Iran for the first time.
Extended-spectrum β-lactamases (ESBLs) are enzymes produced by different species in the Enterobacteriaceae family, among which E. coli is prominent. ESBLs can destroy potent beta-lactam antibiotics such as three and four generations of cephalosporin. Over 600 variants of ESBLs based on amino acid sequences diversity have been identified, of which TEM, SHV, OXA, and CTX-M are the most common (30).
In our study, the prevalence of ESBL producing strain was 46.62%, while in a similar study in Mashhad, the prevalence of ESBL-producing E. coli was 51.6% (31). A systematic review study in Iran estimated that 75% of E. coli producing ESBL has been isolated from UTI infection, and the prevalence of TEM, SHV, and CTX-M genes were 51%, 37% and 45% respectively (32). Based on the results of this study and our results, the prevalence of the TEM gene in Ardabil province is lower than in other provinces. Due to the increase of multidrug-resistant strains and resistance to routine drugs in treating urinary tract infections, proper use of antibiotics seems very necessary. The first-line drugs for the treatment of urinary tract infections are sulfamethoxazole-trimethoprim and nitrofurantoin. This study showed high-level resistance to sulfam-ethoxazole-trimethoprim. As a result, treatment with this antibiotic should be more limited. Thus, it is recommended to use other antibiotics such as nitrofurantoin, ciprofloxacin, levofloxacin, and amoxicillin/clavulanic acid to treat isolates resistant to this antibiotic. In the case of ESBLs strains, their identification should be reported because their treatment with penicillins, cephalosporins, and aztreonam will fail. In various studies, the proposed treatment regimen for these strains is using 4th generation cephalosporins such as cefepime and carbapenems (33).
Today, there are different ways for molecular typing of bacteria, among which ERIC-PCR is the fastest method for differentiating the isolates (34). Although PFGE is a standard gold method for molecular typing of bacteria, previous studies revealed that this method is more suitable, cost-effective, and conve-nient than other PCR-based methods (35, 36).
In our study, ERIC -PCR fingerprinting indicated that despite all isolates causing similar disease, at the genetic level, they are very polymorphic.
In ERIC-PCR analysis, the highest number of isolates was observed in cluster ET2 with 19 isolates. All isolates in this group were resistant to ampicillin and susceptible to nitrofurantoin; they were also isolated from individuals over 70 years old.
To summarize, the results of our study offer valuable information on the prevalence of antibiotic resistance among UPEC strains and provide an appropriate empiric selection of antibiotic therapy based on susceptibility patterns of indigenous strains and local studies.
Treatment of the MDR strains is challenging and costly, and rapid identification of these isolates and preventive strategies are vital. Also, identification of clinical risk factors, suitable drug doses, and therapy duration are essential for managing infections originated by these strains.
This study financially supported by Ardabil university of medical science and presented as a part of an M.Sc thesis (number: 046).
Conflicts of Interest
There is no conflict of interest.
Received: 2021/09/4 | Accepted: 2021/12/28 | ePublished: 2022/01/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |