BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1425-en.html
2- Biologic Research Center, Zanjan Branch, Islamic Azad University, Zanjan, Iran , drreza1357@gmail.com
3- Department of Microbiology, Pasteur Institute of Iran, Tehran, Iran
Pseudomonas aeruginosa is one of the most important pathogens for humans (1). It is an opportunistic, Gram-negative human pathogen (2), especially in nosocomial infections (3), so that the antibiotic resistance development in different parts of the hospital has created issues for its treatment (4).
This bacterium with different virulence factors causes infections of wounds, respiratory and septicemia in the patients with burns and accidents that are associated with a high mortality rate (5, 6). Antibiotic-resistant P. aeruginosa infections are usually difficult to treat. This organism resistance to antimicrobial agents is increasing (7).
Alginate (ALG) is one of the most important pathogenic factors of P. aeruginosa in patients with cystic fibrosis, and its immune response is not dependent on T cells (8). The ALG antigen can be conjugated to exotoxin A (ETA) to increase the production of antibodies in the body (9). ETA is a protein carrier (10) encoded by the toxA gene (11).
Various studies have used a variety of proteins, including pili, outer membrane proteins, and bacterial toxoids, to develop conjugated vaccines for the carbohydrate antigens.
In this study, for the first time, the immunogenicity of ALG-ETA conjugated vaccine was evaluated against P. aeruginosa infection, and the effect of this conjugate was investigated on the IgG antibody titer and its subclasses.
The aim of this study was to bind P. aeruginosa ALG to ETA covalently in order to increase the level of immunity produced by ALG and to evaluate the prod-uction of total IgG, IgG1, IgG2a, IgG2b, and IgG3 anti-bodies against Pseudomonas by ALG-ETA conjugate.
Pseudomonas aeruginosa 6494 mucoid strain obtained from the CF patients and PAO1 standard strain were used for the ALG and ETA isolation, respectively.
Isolation and Purification of ALG
For this purpose, mucoid strains were cultured in the selected medium containing glycerol, dextrose, L-glutamine, Na2HPO4, K2HPO4, and MgSO4.7H2O, and incubated at 37°C for 72 h.
The resulting biomass was then centrifuged for 2 min, and the precipitate was discarded. The supernatant was mixed with 100% cold ethanol overnight and centrifuged at 2500 rpm for 30 min (12, 13).
ALG was converted to a clear solution using 0.1 M Tris buffer, pH 7.5. Then, 0.5% sodium dodecyl sulfate (SDS), 10 mM calcium chloride (CaCl2), and 0.0001 gr proteinase K were added and kept at 56°C water bath for 2 h and 4°C overnight. Then 100 μg/mL DNase and RNase were added and incubated at 37°C for 3 h. ALG was re-precipitated by the addition of cold ethanol. After centrifugation at 4°C, the precipitate, which was pure ALG was sterilized using a 0.22 µM filter (14).
For ALG de-polymerization, it was dissolved in 1% acetic acid and heated at 120°C for 15 min. After cooling, the solution was dialyzed three times in distilled water to remove the fatty acids. The dialyzed solution containing de-polymerized alginate (D-ALG) was mixed with ethanol and stored at 4°C overnight (14).
Preparation of ETA
To prepare ETA, the PAO1 standard strain was used. It was first incubated in Müller-Hinton Broth medium and then in TSBD synthetic medium at 32°C for 24 h on Shaker incubator. Sephacryl HRs 200 gel filtration column was used for purification. In order to detoxify ETA, 5 mL ETA was mixed with 10 mL PBS, incubated at 37°C for 4 days, dialyzed for 48 h, and sterilized using 0.32 µM filter. The toxin was converted to toxoid by heating in the presence of 0.2% formalin for 7 days at 37°C and dialyzed to remove formalin residues (12).
Measurement of Endotoxicity of D-ALG and ETA Samples by LAL Method
D-ALG and ETA samples were dissolved in distilled water at 5 mg/mL concentration and the endotoxin activity of the antigens was evaluated by the PyroMed kit according to the manufacturer's instructions (14, 15).
Conjugation of De-polymerized Alginate with Exotoxin A
For the conjugation, 0.5 M adipic acid dihydrazide, 0.5 M bicarbonate, and 5 mL distilled water was added to the de-polymerized ALG, and after 18 h, the obtained AH-ALG was dialyzed and concentrated. Then, pH was adjusted, 0.1 mg EDEC was added, and re-dialyzed (12). The tubes that contained samples of conjugates showed the most absorption at 210 and 280 nm wavelengths. They were then merged, concentrated, passed through 0.45 µM filter, and stored at -20°C (15).
Purification of D-ALG-ETA Conjugate
To purify ALG-ETA conjugate, it was passed through Sepharose CL-2B Column using gel filtration method. The light absorption of the fractions was recorded at two wavelengths of 210 and 280 nm. The tubes with the highest absorption at both wavelengths were mixed. The conjugates were concentrated by ultracentrifugation, passed through 0.45 µM filter, and stored at -20°C (16, 17).
Pyrogenic Test in Rabbits
To determine the pyrogenic effect of the prepared conjugates, rabbits were selected in groups of 3. After recording the first temperature by placing a thermometer in the anus of the animals, the subsequent temperatures were measured every 15 min for 1 h (The temperature limit was between 38°C and 39.8°C). The sample was then injected through the marginal vein of the ear and body temperature was recorded every 15 min for 3 h. The averages of recorded temperatures were considered. If the temperature rise is less than 0.5°C in each rabbit and less than 1.2°C in three rabbits, the injected substance is a porogen (18).
Toxicity and Sterility Tests
For this purpose, 10 µg of the conjugate samples were injected intraperitoneally to 5 mice, and weight loss and mortality were evaluated in animals. To evaluate the sterility of the conjugate samples, they were cultured at anaerobic and aerobic conditions on thioglycolate, nutrient agar, blood agar, McConkey agar and Saburodextrose agar media, and the culture results were evaluated (19).
Immunogenicity Studies
Sixty female BALB/c mice (6-8 weeks old) were purchased from Razi Research Institute and divided into four groups of 15. The first, second, and third groups were vaccinated with ALG, D-ALG-ETA and ETA vaccines, respectively, each with a dose of 10 µg. The fourth group was injected with normal saline as a control group. Vaccination was performed in three doses (0.5 mL each time) intraperitoneally at two-week intervals and blood samples were taken from 5 mice in each group two weeks after each injection (20).
ELISA Test
In order to obtain the suitable serum dilutions, the cross-titration table method (checkerboard plate) was used. The response levels of total IgG, IgG1, IgG2a, IgG2b, and IgG3 antibodies against D-ALG, ETA, and D-ALG-ETA were measured by ELISA. The detoxification solution was added to the plate and incubated at 4°C overnight. After washing, blocking buffer was added to the wells (300 μL) and incubated for 1 h at room temperature (R.T.). To the first-row wells, 200 μL of serum at 1:10 dilution in PBS was added, and to the other wells, 160 μL of PBS was added. After preparing serial dilutions, the plate was incubated at R.T. for 2 h. After washing, anti-mouse antibody isotypes of goat origin were added to each well (100 μL) at a ratio of 1:1000 and the plate was incubated at room temperature R.T. for 1 h. Following washing, anti-Goat IgG antibodies conjugated with horseradish peroxidase were added to the wells (100 μL) at a ratio of 1:3000 in a wash buffer and incubated at R.T. for 1 h. The substrate solution was then added to each well (100 μL) and the plate was incubated at 15°C for 15 min. Finally, stop solution was added to each well (50 μL) and the amount of light absorption was measured at 450 nm wavelength with ELISA reader (21).
Statistical Analysis
By performing ELISA at three times repeat, the serum titer of each antibody was obtained and presented as mean ± standard deviation (SD). Accordingly, Tukey post-hoc test with P-value<0.01 was used to evaluate the differences in serum titers in the groups receiving different antigens compared to the control group.
Measurement of Endotoxin Activity of De-polymerized ALG and ETA
Measuring the endotoxin activity of de-polymerized ALG by PyroMed kit showed the amount of endotoxin in D-ALG and ETA samples at 0.125 EU/mL. The experiment was repeated with the same results. Regarding the permissibility of the sample for biological use, which should be less than 5 EU/mL, the prepared antigens were used for conjugation.
ALG Conjugation with ETA
In the conjugated molecules, the light absorption peaks of the fractions at 280 and 210 nm coincided precisely, indicating the conjugation of D-ALG polysaccharide antigens to ETA. According to Figure 1, the second small peak observed at 280 and 210 nm was related to non-conjugated ETA and ALG, respectively.
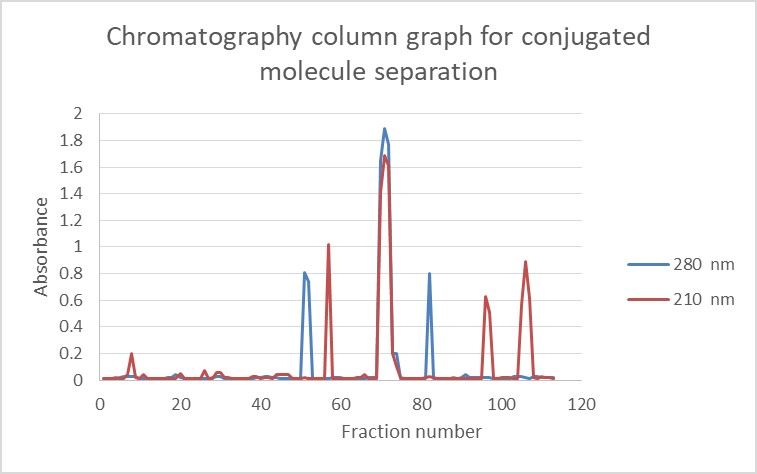
Figure 1. Sepharose CL-2B gel filtration profile of D-ALG conjugated to EXO-A. Fractions were assayed for alginate at 210 nm and 280 nm for ETA.
Pyrogenic Test Result
The prepared conjugate samples were tested for pyrogenic effects in rabbits. After 24 h the temp-erature rise was less than 1.4 in all three rabbits and less than 0.6 in each rabbit. Therefore, the prepared conjugates were free of pyrogen and injectable.
Toxicity and Sterility Tests Results
According to the standard method, the conjugated samples were injected into 5 mice and examined for 7 days. The absence of mortality in animals indicated that conjugates were non-toxic and injectable. On the other hand, examination of the bacterial cultures after 24 h incubation showed sterility of the samples with no growth of microorganisms.
Antibody Titers in Each Group
Examination of the antibody titers obtained at two weeks after the first injection did not show a significant increase in the IgG antibody titer against ALG. Two weeks after the second and third injections, the titers of antibodies in different groups were as follows: ALG-ETA>ALG>ETA (Table 1).
Comparison of Antibody Titers in Different Weeks After Injections
The titers of all target antibodies at two weeks after the first, second, and third injections are shown in Figures 2 to 4, respectively.
Table 1. IgG serum titer against ALG at two weeks after the first, second, and third injections
| Negative control | ETA | ALG | ALG-ETA | |||
| 0.03 ± 0.2 | 0.01 ± 0.1 | 7* ± 109 | 6* ± 401 | IgG | Two weeks after the first injection | Serum titer (OD unit) (mean ± SD) |
| 0.01 ± 0.1 | 0.03 ± 0.2 | 7* ± 74 | 11* ± 120 | IgG1 | ||
| 0.03 ± 0.1 | 0.03 ± 0.2 | 8* ± 28 | 5* ± 48 | IgG2a | ||
| 0.01 ± 0.1 | 0.01 ± 0.1 | 11* ± 21 | 7* ± 40 | IgG2b | ||
| 0.01 ± 0.1 | 0.01 ± 0.1 | 11* ± 74 | 7* ± 119 | IgG3 | ||
| 0.03 ± 0.1 | 0.03 ± 0.1 | 9* ± 393 | 5* ± 1303 | IgG | Two weeks after the second injection | |
| 0.03 ± 0.1 | 0.01 ± 0.1 | 8* ± 140 | 15* ± 475 | IgG1 | ||
| 0.03 ± 0.1 | 0.05 ± 0.2 | 11* ± 160 | 8* ± 105 | IgG2a | ||
| 0.05 ± 0.1 | 0.03 ± 0.2 | 13* ± 30 | 9* ± 89 | IgG2b | ||
| 0.05 ± 0.1 | 0.04 ± 0.2 | 13* ± 115 | 9* ± 465 | IgG3 | ||
| 0.05 ± 0.1 | 0.03 ± 0.3 | 11* ± 410 | 8* ± 2100 | IgG | Two weeks after the third injection | |
| 0.03 ± 0.1 | 0.01 ± 0.2 | 9* ± 191 | 8* ± 1200 | IgG1 | ||
| 0.01 ± 0.1 | 0.03 ± 0.1 | 9* ± 78 | 11* ± 140 | IgG2a | ||
| 0.05 ± 0.1 | 0.01 ± 0.1 | 15* ± 47 | 5* ± 121 | IgG2b | ||
| 0.05 ± 0.1 | 0.01 ± 0.1 | 15*± 180 | 5* ± 1100 | IgG3 |
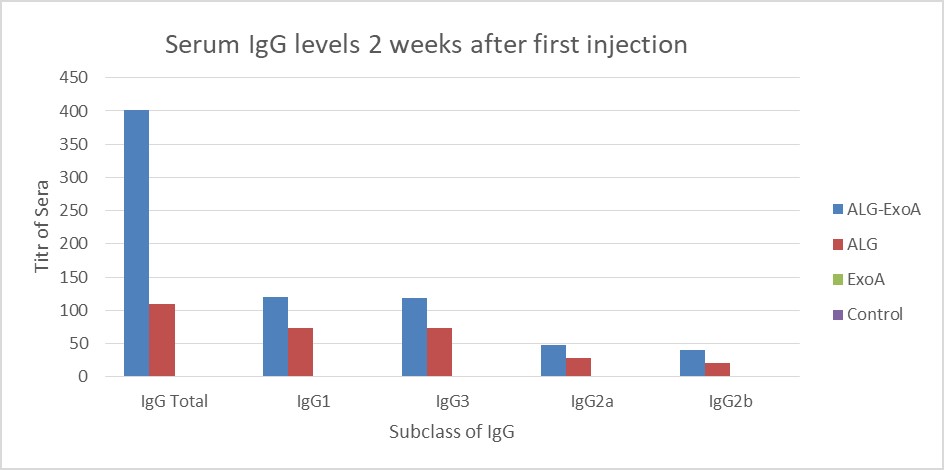
Figure 2. Induction of antibodies in BALB/c mice for two weeks after the first injection (Day 14). The results of inductions for a subclass of IgG antibodies were observed D-ALG-EXO-A> D-ALG> EXO-A
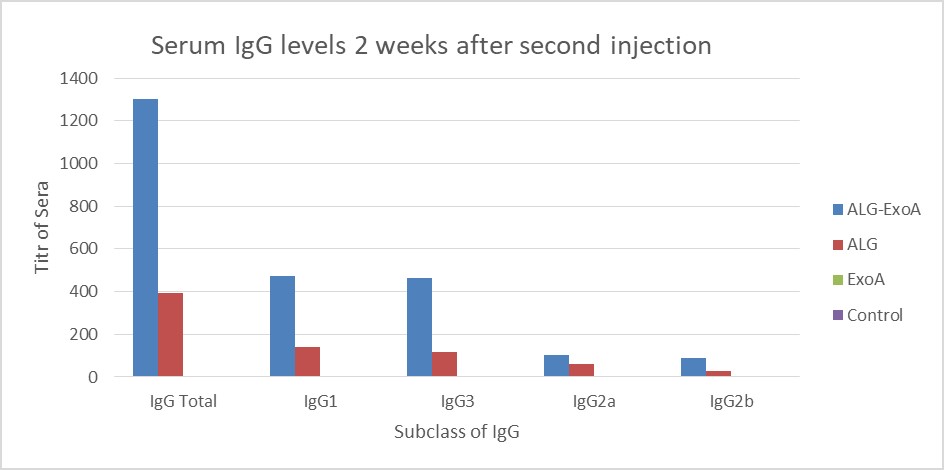
Figure 3. Induction of antibodies in BALB/c mice for two weeks after the second injection (Day 14). The results of inductions for a subclass of IgG antibodies were observed D-ALG-EXO-A> D-ALG> EXO-A
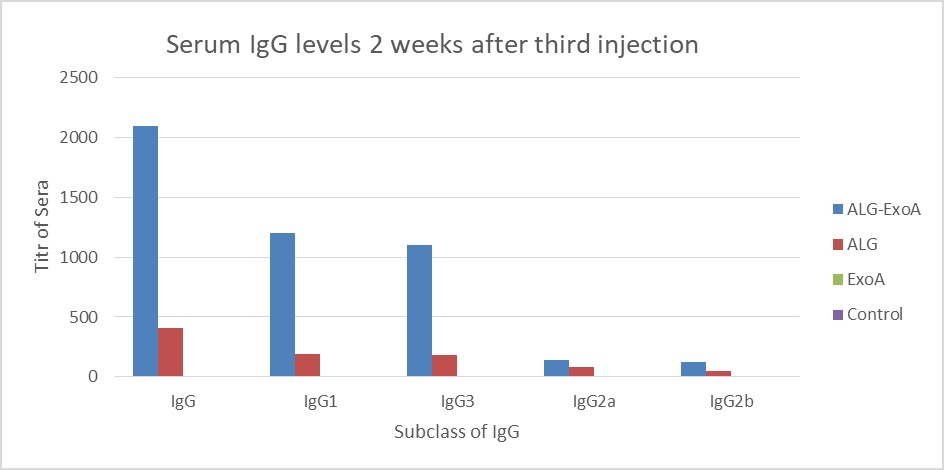
Figure 4. Induction of antibodies in BALB/c mice for two weeks after the third injection (Day 14). The results of inductions for a subclass of IgG antibodies were observed D-ALG-EXO-A> D-ALG> EXO-A
Several studies have been conducted to evaluate the immunogenicity of various conjugates, including ALG-TT, ALG-SLNs, PLGA-ETA, ALG-DT, etc. In this study, for the first time, the immunogenic effect of ALG-ETA conjugate was investigated against P. aeruginosa infections. The effect of this conjugate on IgG antibody titer and its subclasses was also evaluated.
The ETA is one of the most important pathogenic factors of this bacterium and is produced by most of the clinical strains of P. aeruginosa. It has immunogenic effects and similar function to diphtheria toxin (7). ETA antibodies have protective effects against P. aeruginosa infections and have been used as a protein carrier in P. aeruginosa polyvalent vaccine candidates (21, 22).
The ETA secreted by P. aeruginosa is a toxic and lethal pathogenic factor that inhibits protein synthesis by the host cells. It also causes tissue changes in the liver, apoptosis in hepatocytes, and increased expression of proinflammatory cytokines.
Research has shown that these destructive effects are counteracted by the presence of anti-ETA antibodies, and all vaccines containing ETA toxoid prevent death. Therefore, these results indicate that the use of a combination of non-toxic ETA along with other antigens in vaccines is very useful in eliciting an effective immune response (23).
Due to the advantages mentioned in this study, we conjugated ALG with ETA. In addition to being non-toxic and non-pyrogen, this conjugated vaccine can produce functional antibodies against ALG and ETA. In other words, antibodies are produced against two important pathogenic factors of this bacterium, and this will increase the immunogenicity and efficiency of the vaccine (22).
The purpose of this study was to produce a vaccine by conjugating ETA as a protein moiety to the surface carbohydrate capsule antigen using modern methods to provide long-lasting protection against Pseudomonas infections.
ETA was used as a protein carrier for the polysaccharide molecule, and the ALG was used as an adjuvant to induce greater immunity compared to ETA alone so that if proven effective, these conjugates could be introduced as highly effective candidate vaccines.
All the ALG-ETA and ETA groups showed a significant difference in serum antibody titers in all three injection periods (two weeks after the first, second and third injections) compared to the control groups.
A study by Creeze et al. in 1997 on the ALG conjugated with tetanus toxoid (TT) protein as a protein carrier showed that more IgG antibodies were produced against conjugated ALG along with a protein carrier (24).
Also, in 2006, Kashif et al. co-conjugated ALG with TT. The results showed an increase in IgG titer in the conjugated form. This vaccine also helped mice survive the deadly mucoid strains of P. aeruginosa. One of the disadvantages of TT conjugates is that repeated usage of TT in high doses in human vaccines can over-stimulate the immune system, which can prevent the global use of TT as a protein carrier. In this study, antibodies were produced against ALG and TT (25). By replacing TT with ETA in our study, functional antibodies were produced against ALG and ETA simultaneously, which increased the effectiveness of this vaccine.
In another study by Lang et al. in 2004, LT-B E. coli toxin and P. aeruginosa exoprotein A (rEPA) were used as carriers (26).
In the present study, conjugated ALG immunization was higher than pure ALG, the same as Lang's study. So that, the titer of IgG antibody produced against ALG in the third injection was increased by 5.2 times compared to the first injection of the conjugate.
According to similar studies, ALG-ETA conjugate of P. aeruginosa produces the best antibody titer against polysaccharides (16, 21, 27, 28).
Consistent with the results of this study, Afshari and colleagues used SLN (solid lipid nanoparticles) to enhance the immunization of ALG. In this study, the titers of all antibodies against ALG-SLNs conjugate were 6% higher than mice tested with ALG alone (29). The highest antibody titer was related to IgG. In this study, the titer of IgG antibodies against ALG-ETA conjugate was higher than ALG alone, and the titer of IgG antibodies increased against ALG.
In 2015, Najafzadeh et al. evaluated conjugated lipopolysaccharide-diphtheria toxin and ALG-diphtheria toxin vaccines. In this study, D-LPS and D-ALG were covalently linked to DT as a protein carrier by amidation. The results showed that the IgG antibody of mice immunized with D-LPS-DT conjugate was 4 times higher than that of mice vaccinated with D-LPS alone. Their results also showed that the conjugated vaccine based on D-ALG P. aeruginosa and DT had a greater increase in antibody titer than D-LPS-DT. This indicates that ALG provides better immunization.
We also used ALG in our study. Comparing the results of D-ALG-DT and D-ALG-ETA conjugate vaccines shows that D-ALG-ETA causes a further increase in the titer of anti-ALG antibodies. So that, after the third injection dose, the IgG titer increased slightly above 1800 in D-ALG-DT conjugate and to 2100 in D-ALG-ETA conjugate. Therefore, it can be concluded that as a protein carrier that is part of bacteria, ETA can act better in the immunization compared with diphtheria toxin (30).
In the present study, by adding a protein compound to the polysaccharide fraction, the titers of antibodies IgG2a, IgG2b, IgG, IgG1, and IgG3 produced against ALG increased by 2.9, 3, 5.2, 10, and 9.2, respectively.
In a 2019 study, Safari Zanjani et al. evaluated the immunogenicity of ETA conjugated with PLGA nanoparticles as a vaccine candidate in P. aeruginosa. Based on the results, ETA-PLGA could increase IgG responses to ETA antigen in the immunized mice as a suitable immunogen (9).
In the present study, the titer of IgG antibody and its subclasses also showed a significant increase after the third injection dose in the vaccinated groups with ALG-ETA compared to the vaccinated group with pure ALG. Among different protein carriers, only diphtheria and tetanus toxoid carriers have succeeded in obtaining a vaccine license (31).
Our findings showed that antitoxin A antibodies can play a greater protective effect than P. aeruginosa infections. This conjugated compound increases the level of anti-ALG IgG antibodies, and immunization with this type of conjugate can stimulate toxin A-neutralizing antibodies. This ALG-based conjugated vaccine is non-toxic, with no pyrogenic effect, producing functional antibodies against ALG and ETA. Another feature of this conjugate is the stability and activation of the antigenic structure. Regarding the effectiveness of these vaccines in research, it can be suggested that the resulting conjugated vaccines be introduced as a suitable candidate vaccine against Pseudomonas diseases. The study also found that ETA could act as a protein carrier in conjugated vaccines with good stability.
Various studies on the immunogenicity of ETA have all shown that antibodies produced against ETA can prevent death. Thus, these results suggest that using a combination of non-toxic ETA with other antigens in vaccines is very useful in eliciting an effective immune response.
None
This article is an independent study conducted with no organizational financial support.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2021/08/11 | Accepted: 2021/12/30 | ePublished: 2022/01/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








