BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1362-en.html
2- Pathology and Stem Cell Research Center, Department of Pathology, Afzalipour School of Medicine, Kerman University of Medical Sciences, Kerman, Iran
3- Gorgan Pathobiology Laboratory, Tehran, Iran
4- Reference Laboratory, Health Reference Laboratory (HRL), Ministry of Health and Medical Education, Tehran, Iran
1. Architectural Design in a Medical Laboratory
Laboratories are made up of various structural dimensions from space, equipment and small number of staff to large laboratories including various spaces, specialized manpower, and various equipment. Accordingly, the design of laboratories should be formed based on the needs, facilities, manpower, equipment, and available space (1).
The laboratory design includes pre-design according to the user needs, situations and accesses according to the context, flexibility of the space for the future development, equipment, staff and various activities in unpredictable cases, safety and increase in the productivity including biosafety cabinets, fire protection and prevention systems, emergency shower, well-designed exits, proper ventilation and temperature control systems, and finally physical planning (1).
Observing the minimum conditions and standards necessary for the design and implementation of a medical laboratory will not only create a suitable space for the staff and clients, but also increase the productivity by optimizing the affairs. The shortcomings and mistakes in this context even minor can cause irreparable damages.
This study tries to examine the dimensions, requirements and standards in the field of medical laboratories design. The identifying damages and safety protocols as well as the risks involved in this case are not within the scope of this study.
2. Research Method
The methodology of this research was based on the descriptive and analytical method. In this research, the design of the space was demonstrated, by collecting the articles and reliable sources regarding the physical design of the laboratories. The collected sources were analyzed and cited.
3. Components and Stages of Architectural Design in a Medical Laboratory
3-1- Pre-design
One of the main stages of designing laboratories is pre-design. At this stage, the overall design is done with flexibility in the use of spaces and the possibility to move if necessary. The designer must also consider the factors such as adapting the laboratory environment to different people and devices. Compliance with the principle of flexibility in predicting the infrastructure of mechanical installations (type and capacity of units and access to the risers and so on) and electrical installations (system capacity and location of sockets) in order to allow for the intended displacements, resolves this problem well (Figure 1).
Figure 1. Schematic diagram of laboratory workspace for the mobile and flexible workstations (2).
3-1-1- Space Design and Requirements
3-1-1-1- Proximity of Space
The related technical section in a medical laboratory should be integrated and adjacent to each other or at least as close as possible, while some laboratory spaces should be separated from other sections. The following three conditions can be considered for the proximity of spaces in the laboratory:
-
Adjoining Spaces
For adjoining spaces, technical sections such as biochemistry, hematology, immunology and serology can be mentioned.
-
Adjacent Spaces
For adjacent spaces, the proximity of the reception space, sampling and sample separation can be mentioned.
-
Separated Spaces
For separated spaces these sections can be mentioned: washing and sterilization spaces, refrigerated and non-refrigerated storages, server and central UPS rooms, dining and rest room, staff locker room and technical departments including microbiology, urology, mycology, parasitology, virology, molecular laboratory and others (3).
3-1-1-2- Requirements in Meeting the Mobility Needs of People with Disabilities
Paying attention to the mobility needs of the disabled people in the city and building scale is one of the minimum standards of the developed countries, which in our country has not received much attention. This issue should be considered for the patients, clients and laboratory staff (3).
Among the points and requirements related to the mobility needs of the disabled people these items can be pointed. For example, the possibility of movement in all the main spaces of the laboratory, easy access considering the spaces dimensions, net width of the entrances and corridors and elevator cabs (with built-in handrails), installation of laboratory tables with the ability to height adjust for the people in wheelchairs, the use of laboratory hoods with the ability to use while sitting, adaptation of toilets, and designing a recall or audio pager system suitable for the people with hearing problems.
Obviously, the number of these facilities at the laboratory level should be determined in proportion to the number of staff and their daily admission. These cases should be designed in a way to allow people with disabilities to enter and exit easily in emergency situations (4). Figure 2 shows an example of a space design for the people in wheelchair (5).
Figure 2. Minimum dimensions of desktop design for the disabled and elderlies (5).
3-1-2- Quality of Medical Laboratory Space
The most important factor in providing quality in a space depends on the appropriateness of the space by the user type. This proportion does not only include the quantity and size of the space, but a set of factors such as light, ventilation, electricity, water and sewage, the equipment arrangements, the type of steps and platforms, and safety levels all play roles in shaping the overall quality of a space. The existence of unnecessary columns, walls and partitions can reduce the quality of a space (6).
3-2- Physical Planning
3-2-1- Physical Planning of Laboratory Spaces
In the design process, after pre-design, the attention is paid to the physical planning of the space. In physical planning of laboratory spaces, the required area is determined by listing the required spaces, their user description and their communication system (5, 3).
3-2-2- Quantity and Separation of Medical Laboratory Spaces
Mostly, the minimum physical space that seems sufficient at the opening of a laboratory becomes inadequate and inefficient with the development and growing process of the laboratory and the definition of new experiments and equipment and also new technical departments and staff. Sometimes even to provide a little space needed, the narrow corridors of the laboratory are inevitably used for the technical applications. Sometimes, due to financial and economic constraints or lack of prediction and information about the expansion and development of laboratory activities in the future, the minimum space is considered for the initial space. In these cases, this space should be provided in a place where side spaces or the other floors should be available in its vicinity. With a detailed study and comprehensive information, the relative area needed to expand the space in the future should be considered (2).
At present, according to the regulations for the establishment and administration of medical laboratories, the minimum space that fits for the type of laboratory activity is 120 m2 for the clinical and descriptive pathology laboratories, 100 m2 for the clinical laboratories and 60 m2 for the single specialized laboratories (4). A suitable space should be provided for the work in the laboratory to ensure the quality, safety and efficiency of the services, and the health and safety of the staff, patients and clients. The suitability and suffice of the dedicated spaces should be evaluated and determined for the activities (4).
Insufficient work space or improper design of physical space can be potentially the source of many laboratory accidents and hazards and endanger the safety of staff, such as the physical contact of staff with each other during work (with the risk of contamination with infectious, chemical and hazardous substances), or the staff contact with equipment that could result in physical injury to the staff and damage to the equipment. Therefore, the design of a laboratory should include the design of the required sections, their area and their arrangements.
3-2-3- Main Sections of Medical Laboratory
Every medical laboratory needs different departments. These sections include the client lounge, reception and cashier, sampling section, blood bank, supervisor room, etc. Figure 3 depicts an ideal image of a medical laboratory parts.
Figure 3. Schematic view of different laboratory spaces based on HMI index (2).
3-2-4- Separation of Physical Space
Separation and organization of physical space is the most controversial and important issue in the laboratory design that should be defined by considering the overall dimensions of the laboratory, the number of staff, the volume of technical operations, the type of experiments, and the size of equipment in each technical department (7).
Some spaces must be defined isolated from other laboratory spaces, including the microbiology, mycology, washing, and sterilization sections. In addition, office and sampling spaces should be separate from other spaces. The corridors between workspaces should be designed in such a way that the staff safety in the work space is maintained. The corridors should be wide if possible (8).
The minimum standard width of corridors and laboratory tables according to the laboratory design guide (GP18A2) is one and a half meters, which is shown in Figure 4 (8).
Figure 4. Minimum width of laboratory tables according to the laboratory design guide (6).
3-2-5- Controlling and Monitoring the Laboratory Physical Space
The design of physical space control systems is very necessary, in addition to the quantity and quality of the space. They include the design of audio and video systems and CCTV to control the number of active staff, movement and congestion of the patients and workflow time, the workflow of samples in workspace especially emergency samples, staff entry and exit, and also internet control from remote distance.
3-2-6- Add or Remove Physical Space in the Laboratory
Ideally, as the need for the space in the laboratory may increase or decrease, there should be possibility to expand or limit the physical space of the laboratory. In order to develop the space, it should be anticipated that potential lateral spaces of the laboratory will be available and usable (ability to buy or rent).
3-2-7- Movement in the Medical Laboratory Physical Space
Assessing the movement of staff and patients and clinical samples in the physical space is better to be drawn in a map and diagram and movement restrictions for each group to be specified; such as restricting the movement of patients to the technical spaces and restricting the movement of staff in specific spaces (for example, unskilled staff movement in the mycobacteriology or specialized microbiology or molecular department will not have any other outcome except for the contamination of the patients samples and the occurrence of false positive results and contamination of the staff). To draw this diagram, it is suggested to first magnify the laboratory spaces (in the map) and conduct the necessary analysis to design the spaces, including location, entrance of the workstations, equipment location and other items. The real map can be drawn after simulating this map (1).
3-2-8- Sample Transfer System Design using Compressed air (Pneumatic System)
The design of the sample transfer system using the compressed air has been considered in the developed countries. By designing this system in hospitals, laboratory samples can be easily transferred from the clinical wards of the hospital to the sample isolation unit and from the isolation unit to the technical wards. In independent central laboratories, samples will also be transferred from the sampling unit to the isolation and technical departments. In general, the advantages of this method include quick and easy access, reduction of traffic between different departments, cost reduction and sample safe transfer.
3-2-9- Location and Position of the Equipment in the Physical Space
In the list of defined equipment in each physical space, except for the exact position and location of each piece of the equipment, the following items must be precisely defined and recorded (9):
-
Dimensions of the equipment or device (length, width and exact height of the device) along with the minimum lateral space required for each equipment (distance from the wall and sides)
-
Definition of the dimensions of the necessary peripherals for each device such as monitor, printer and UPS (preferably these peripherals should be placed in the space on the wall or behind the cabinet to provide more space for the work area)
-
Definition of the power sources (required voltage and current for the device) and water and sewage system of the device
-
Definition of the way to access the back and side spaces of the device during the service of large and bulky equipment (for example, it is better to place large and bulky equipment on a separate wheeled table so that, the service person can easily access the side and back spaces of the device with the least displacement and the least energy consumption).
-
Prohibition of proximity of some equipment (for example, prohibition of proximity of centrifuge and microhematocrit with the cell counter, etc.)
-
No proximity of the heat-sensitive equipment to the laboratory heating systems (especially ELISA desk).
-
Keeping a minimum distance of 20 cm for the refrigerator to the wall for the heat exchange
-
Suitable location for the equipment that generates high heat (a place with air circulation capability to prevent heat retention and damage to the equipment) and proximity of these equipment to the standard cooling systems
3-2-10- Laboratory Furniture Design
The laboratory furniture should have smooth and non-porous surfaces to resist the absorption of liquids and the severe effects of disinfectants. The furniture should not be positioned in such a way that makes cleaning the spilled liquids or performing routine tasks difficult. For example, placing a Class II biosafety cabinet in a confined concave space may not allow the biosafety cabinet certification to verify the cabinet panels upon re-inspection. These resources are applied specifically to the laboratories containing biological and radioactive materials (11).
Many common materials in the laboratory are chemical solvents such as formaldehyde, phenol, and ethanol, which are considered corrosive substances. The laboratory cabinets and benches must be resistant to the chemical reactions of these materials. Wooden bench covers are not suitable for this purpose because a wooden surface can absorb the liquids. Also, wood burns quickly in the event of a fire. Fiberglass is also unsuitable because it can be damaged by the use of strong disinfectants. Fiberglass also releases toxic fumes when burned (12). One of the factors that is suitable for the laboratory, is the use of sufficient natural or artificial light to ensure sufficient visibility for operational safety (13).
3-2-11- Physical Space Required by the Medical Laboratory Staff
The staff are one of the main and important elements of any medical laboratory. Thus, proper design of work space and their movement in the lab environment and the level of their safety in this space will be particularly effective in the staff satisfaction and final quality and overall performance of the laboratory.
The calculation of the total (technical) working space of the medical laboratory (TNS: Total Net Space) is obtained from the following equations:
TNS = BWS + FMI
BWS= [(AE + CWS) x (CD+AW)]
The abbreviation of the different physical spaces of the medical laboratory used in this formula is given in Table 1.
3-3- Safety, Increased Productivity and Necessary Equipment
3-3-1- Biosafety Laboratories and Their Requirements Design
Classification is based on the biosafety levels to provide information on how to protect staff and the laboratory environment against laboratory contaminations in order to achieve acceptable standards and guidelines. This information is also used to protect the health of laboratory processes by controlling the spread of various contaminants. In fact, the main purpose of the biosafety discussions in laboratories is that no experiment is more important than the safety. Therefore, it is necessary to plan and control bio-contaminations to protect against laboratory infections and control the spread of contaminants in laboratories that use contaminated and hazardous materials. Obviously, determining the type of laboratory in terms of biosafety levels can affect the way the laboratory is planned and designed.
Table 1. The abbreviation of the different physical spaces of the medical laboratory (8)
| TNS: Total Net Space | Total technical (working) space required for the laboratory |
| BWS: Bench Work Space | Work space of laboratory platforms and tables |
| FMI : Floor Mounted Items | Items attached to the laboratory floor (either fixed or glued) |
| AE : Analysis Equipments | Laboratory equipment space |
| CWS : Clear Working Space | Clean space (without accessories) of laboratory |
| CD : Counter Depth | Depth (height) of counters |
| AW : Aisle Width | Side spaces (corridors) |
In other words, biosafety is a widespread issue. Biosafety agents include personal protective equipment (PPE), four levels of biosafety cabinets, disinfectants, diagnostic methods including identification, evaluation and control of a wide range of microorganisms and environmental hazards, pathogens, viral agents, fungal agents, common human-animal agents, protozoa, and gene transfer vectors and biorisk management including regulations and guidelines for the risk assessment covering pathogenic microorganisms, allergens, toxins, aerobics, occupational medicine, and biological safety (14).
According to the definition of the World Health Organization (WHO), laboratory biosafety refers to the application of restrictive principles, technologies and methods to prevent the unintentional release of pathogens and toxins into the environment. The laboratory biosafety is applied to the set of security measures and methods used by the staff and organization to prevent the loss, theft, misuse, diversion or intentional release of pathogens and toxic substances. In the case of laboratory biosafety, in addition to physical protection, measures such as personnel management, material control and auditing, information protection, and transportation security should also be considered. Considering the above concepts, the architects and designers of the health centers should pay attention to these points in designing laboratories or hospitals (15).
3-3-1-1- Biosafety Laboratory Level 1 and Its Requirements
The level 1 laboratory is suitable for working with microorganisms with low risk level and laboratory personnel are adequately protected in accordance with the laboratory standards. The organisms tested in these types of laboratories do not cause disease in healthy people, and laboratory processes may be performed outdoors (outside the hood). Ventilation of this laboratory can be provided naturally.
Level 1 biosafety represents laboratories that have a basic level of safety and are based on the use of standard methods in disinfection. These laboratories are usually at the lowest level of biosafety, and precautions including regular hand washing and the use of protective equipment such as gloves, a separate wash sink, and appropriate disinfectants to disinfect surfaces and hands are necessary. The media containing microbial culture and residues should also be autoclaved (15).
3-3-1-2- Biosafety Laboratory Level 2 and Its Requirements
The level 2 laboratory is suitable for working with materials containing microorganisms that are associated with human diseases and have the ability to infect by ingestion and exposure to mucous membranes and are present in the environment. Using appropriate microbiological methods, the experiments can be performed out of hood. However, if there is a risk of airborne particles, a biosafety hood should be used.
These laboratories include the health service laboratories, and diagnostic, research, and training laboratories. In these types of laboratories, a wide range of microorganisms with moderate risk are worked that exist in the community and have different pathogenicities with low risk.
In level 2 laboratories, if aerosols (particulates in the air) are slightly formed during laboratory processes, in other words, if the risks of laboratory processes are low, they can be performed on benches without protection, but if these processes produce large amounts of aerosols and increase the risk of contact with these agents, biosafety hoods should be used.
3-3-1-3- Biosafety Laboratory Level 3 and Its Requirements
The level 3 laboratory is suitable for working on native or non-native pathogenic microorganisms that have the potential to be transmitted by the air and are dangerous to inhale or cause deadly disease. Pathogenic microorganisms in this group include MERS-COV, SARS-CoV-1, SARS-COV-2 and similar organisms (15).
Given the potential dangers of these pathogens, there are criteria for setting up a laboratory that manages the work and has the facilities to reduce the risk of microorganisms for human and environment. At this level, laboratory experiments are performed in a confined and controlled space (4). These laboratories include specialized diagnostic and research laboratories. Level 3 biosafety represents laboratories that require the use of biosafety cabinets or hoods in accordance with the international standards and secondary protections such as negative air pressure systems.
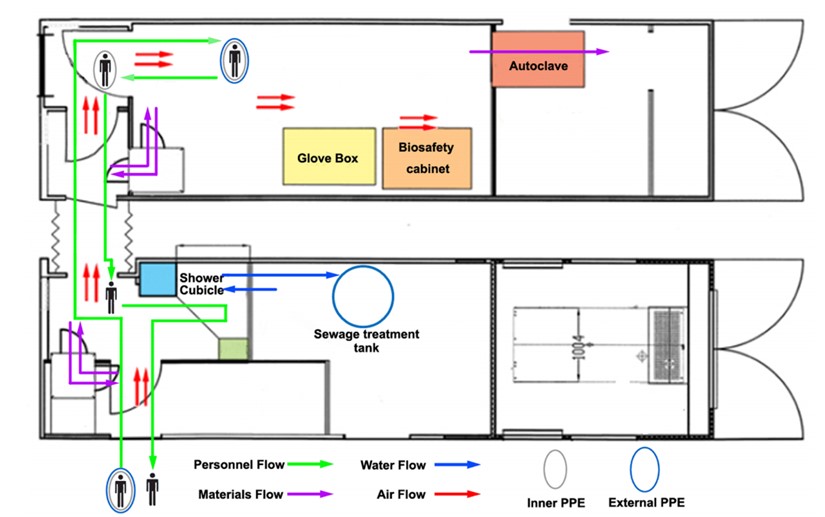
This level of laboratory in terms of laboratory operations includes all the requirements of level 2 in addition to the special clothing and coverings as well as controlled access (entry and exit restrictions) as well as directional airflow. In terms of safety equipment, most processes are performed in biosecurity hoods. A schematic view of a level 3 biosafety laboratory including the workspace, air, water and materials circulation, protective equipment, and entry and exit of individuals is shown in Figure 5 (16).
Figure 5. Schematic view of the hood location and air, water and materials circulation and the entry and exit of staff in the level 3 biosafety laboratory (12).
3-3-1-4- Biosafety Laboratory Level 4 and Its Requirements
The level 4 laboratory is suitable to conduct dangerous experiments with life-threatening microorganisms that spread rapidly through aerosols in the community. In general, the laboratories where the virus is cultured are level 4, and this level of laboratory requires special requirements that must be considered in a separate building. In terms of the type of laboratory, it includes diagnostic units that are designed to detect dangerous pathogens (culture of microorganisms). These laboratories work with microorganisms that are transmitted through respiration and there is currently no suitable vaccine or treatment for them (4).
Dangerous and life-threatening pathogenic microorganisms of this group include viral hemorrhagic fever such as Marburg virus, Ebola virus, Lassa virus, Crimean Congo hemorrhagic fever and similar cases (4).
Level 4 biosafety represents laboratories that are considered very limited in terms of biosafety level and their building is located in a completely isolated area. The use of negative pressure system, shower and air lock are mandatory and are of great importance and sensitivity.
In terms of laboratory operations, it includes level 3 requirements along with air locking system at the entrance, shower at the exit, as well as special management for the proper waste disposal.
In terms of safety equipment, it consists of the use of Class 3 biosafety hood or positive pressure clothing with Class 2 biosafety hood, as well as a two-door autoclave (located between the walls) to remove material and filtered air (1). A schematic view of the four levels of biosafety and their equipment and facilities is shown in Figure 6 (17).

Figure 6. A schematic view of the four levels of biosafety and their equipment and facilities is shown in Figure 6 (17).
3-3-2- Proper Ventilation and Temperature Control Systems
Mostly, air conditioning is perceived as cooling and heating, but it is important to know that it is not just about cooling or heating. Using air conditioning technology, the humidity, air flow rate, air pollution and air speed can be controlled. In other words, with the help of air conditioning, even airborne particles and air pollution can be eliminated. It removes excess moisture from the air and automatically maintains the amount of heat or cold required by the environment to provide comfort and relaxation for the people in the work and living environment. Air conditioning has not only general applications but also usage in sensitive areas such as gas disinfection rooms or special material storage environments (18).
3-3-3- Safety Regulations for Working with Recombinant DNA
Recombinant DNA technology involves combining genetic material from different sources to create a genetically modified organism (GMO) that has never existed in nature. There are always concerns about undesirable and unpredictable properties of such organisms, especially if released suddenly in nature (20). The nature of the manipulated organism, the nature of the gene fragment transferred, the characteristics and conditions of storage, and working with the new organism are very important. A genetic order may give a new and unknown characteristic to the host organism. Therefore, observing the safety principles in all stages of genetic manipulation is necessary to minimize the negative effects of these studies (19).
3-3-3-1-Working with Expression Systems to Produce Recombinant Proteins
The expression systems include a host organism and a vector. The desired genes are cloned into the vector and transfected to the host. Escherichia coli is one of the most common bacteria used as an expression host. It is a non-pathogenic bacterium for the healthy humans and animals. After performing the genetic engineering manipulations, it is necessary to observe the safety principles (20).
If there is no accurate information about the input DNA fragment, it should be handled with extreme caution, like the condition when the genetic library is made from the genome of a pathogenic organism.
More caution should be exercised if the input gene product is toxic or has pharmacological and therapeutic effects (20).
3-3-3-2-Working with Viral Vectors for Gene Transfer
Viral vectors such as adenoviruses and other similar viruses are widely used to transfer genes to the cells. Such viruses lack some of the genes involved in reproduction and replication, and can replicate in the cells that make up this defect. Such viruses may regain their lost potential through recombination with other viruses or host cells. Therefore, it is necessary to always observe all the principles of safety working with complete viruses (21).
3-3-4-Biosafety Cabinets (Hoods)
The biologic safety cabinet (BSC) is a laboratory workspace that is used safely for the contaminated material or suspected pathogens. Biosafety hoods are designed as a reliable tool to protect the user, laboratory environment and work space against aerosols, contaminated discharges when working with infectious substances, and working with chemicals and radioactive materials.
Usually in different types of hoods (cabinets) biosafety is provided by the high efficiency filters against particles (High Efficiency Particle Air: HEPA) or filters resistant to fine particles (Ultra Low Particle Air: ULPA) at the system outlet (exhaust) or an air supply is used to trap aerosols. These filters are able to trap 97.99% of particles with a diameter of 0.3 µm and 99.99% of particles with larger diameters. Therefore, they create a safe environment in the laboratory.
Depending on the structure of these filters, the direction of airflow and the height of the window, different levels of protection of staff, product and work environment can be achieved.
The selection of an appropriate biosafety hood is based on the risk assessment of the types of biological organisms and other materials used.
3-3-4-1-Types of Biosafety Cabinets and Their Working Method
Biosafety hoods have been developed in three categories/classes 1, 2 and 3 based on the different needs of diagnostic and research centers.
Class 1 biosafety hoods have a filter located at the outlet that only protects the user from the chemicals and unpleasant odors. Most of these hoods have a HEPA filter, which returns the air to the environment after purifying. In Class 1 biosafety hoods, room air enters the interior part of the hood through the open front area and passes through the working surface and is discharged through the air outlet duct. Usually in these types of hoods, the room air enters the working surface at a speed of at least 38.0 m/s and then enters the air outlet duct. Figure 7 shows a schematic of the operation of a Class 1 biosafety hood (22).

Figure 7. Schematic view of Class 1 biosafety cabinet operation (13) A: Front opening, B: Sash, C: Exhaust HEPA filter, D: Exhaust plenum.
Figure 8. Schematic view of Class 3 biosafety cabinet operation (13).
Due to the need for cell and tissue culture to propagate viruses and so on, the passage of non-sterile air over the work surface causes contamination. Therefore, Class I biosafety hood is not applicable in these cases. For this purpose, to protect the work surface from the polluted room air, Class 2 biosafety hood was designed, in which sterile filtered air by HEPA filter flows on the work surface. Therefore, these hoods are used for cell and tissue cultures.
The Class 2 biosafety hood is the most common type of hood in a medical laboratory. These types of hoods protect the product in addition to the user. Basically, the rotation of indoor air is away from the user and does not return to him. When using this type of hood and working with hazardous chemicals, the hood with outlet duct (channel) must be used (1). Class 2 hoods can be used to work with the infectious agents in danger groups of two and three.
Class 2 hoods are produced in four different subcategories (types) of A1, A2, B1 and B2 based on the needs of the centers. Their difference is based on the air intake speed, the amount of air returning to the working surface, and the performance of the system determining the amount of exhaust air.
Class 3 biosafety hoods provide the highest level of employee protection and are suitable for working with danger group of four. All their pores are sealed and insulated to prevent any uncontrolled air flow to the hood. Access to the work surface is possible only through thick rubber gloves attached to the hood and insulated. In some types of these hoods, it is possible to use a two-door autoclave to decontaminate all contaminated materials and debris entering or leaving the hood. Class 3 biosafety hoods are suitable for work in levels 3 and 4 biosafety laboratories.
In Class 3 biosafety hoods, the air outside the hood first passes through the HEPA filter and then enters the hood. This purified air, after circulating inside, passes through the other two HEPA filters again and leaves the hood (1). A schematic of the operation of a Class 3 biosafety hood has been shown in Figure 8 (13).
3-4- The Flexibility of Space for Future Development
Advances in technology in medical laboratories and changes in requirements raise the need to anticipate changes in user space and future development in spaces. One of the things that can meet this need is the use of flexible partitioning systems with the ability to move and separate parts, which should be seriously considered in the design of the laboratory.
One of the problems, especially in hospital laboratories, is the surrounding of laboratory spaces with other parts of the hospital (23) that make it difficult to develop spaces. This problem should be considered in the initial design of hospital centers, so that, the desired environment in the development of hospital centers and consequently the laboratory will face fewer challenges.
The design of medical laboratories, taking into account the issues mentioned in this article, will lead to the construction of a place that will not only meet the needs of clients but also provide comfort and safety for the staff. Such a laboratory will be more profitable by increasing the productivity, which will ultimately lead to a comprehensive profit. Errors in the design and execution of any of these items can cause minor and major damages which are beyond the scope of this discussion. As stated in this article, the design steps include pre-design, physical planning, safety and required equipment efficiency, and the flexibility of the space for future development, which should be considered in the design of the laboratory physical space. In addition, these steps themselves include more detailed steps and requirements and standards that must be observed in the design of the physical space of the laboratory.
Not Applicable.
This article is an independent study that was conducted without organizational financial support.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2021/06/12 | Accepted: 2021/08/10 | ePublished: 2021/09/15
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |