BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1235-en.html

 , Mohammad Reza Zolfaghari2
, Mohammad Reza Zolfaghari2 
 , Salehe Sabouri Shahrbabak3
, Salehe Sabouri Shahrbabak3 
 , Mohsen Zargar1
, Mohsen Zargar1 
 , Mohammad Soleimani4
, Mohammad Soleimani4 

2- Department of Microbiology, School of Basic Sciences, Qom Branch, Islamic Azad University, Qom, Iran ,
3- Pharmaceutical Biotechnology, Faculty of Pharmacy, Kerman University of Medical Sciences, Kerman, Iran
4- Department of Microbiology, Faculty of Medicine, AJA University of Medical Sciences, Tehran, Iran
.
oof the most significant problems in global health in the last decade has been the increasing prevalence of antibiotic-resistant infections, including gram-positive and gram-negative bacteria, specifically the "ESKAPE" bacteria (i.e., Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) (1, 2). Excessive usage of antibacterial drugs might result in elevated multipledrug resistance (MDR) infections and changes in the bacterial population in patients (3).
Gram-negative bacteria, such as E. aerogenes and K. pneumonia are resistant to cephalosporin, penicillin, and carbapenem family leading to MDR infection formation. E. aerogenes and K. pneumoniae can express carbapenemase (KPC), extended-spectrum beta-lactamase (ESBL), and metal beta-lactamase that have not been defined as a class of antibiotics capable of controlling the infection (4-7).
Gram-negative MDR contamination in individuals with poor immune systems, such as infants, elderlies, immunosuppressed cases, and cancer patients, is a real danger that results in extreme neutropenia symptoms and even death in some cases (3, 8). The rising prevalence of MDR infections, treatment difficulties, and extended therapy has led to the production of high-efficiency new antibacterial medications with lower side effects and low cost for the management of MDR infections.
An alternative that has gained a lot of interest in the last two decades is bacterial viruses or bacteriophages discovered almost a century ago (9, 10). One type is lytic phages that enter into bacterial cells and change bacterial metabolism causing bacteriolysis and destruction (9, 11). Since the early 20th century, phage therapy has been used as an agent for the treatment of infections caused by both gram-positive and gram-negative bacteria (9-11).
Considering the benefit of phage therapy, several European countries and the United States have started to discover new bacteriophages for the antibacterial management of drug-resistant infections and seriously infected patients (12-14). Some preferences of phage therapy entail efficacy in treating MDR infections, not destroying the natural microbial flora of the patient, lower costs of phage therapy relative to broad-spectrum antibiotics, fewer side effects, stability in unfavorable temperature and drought conditions, specificity for the target bacteria, lacking toxin genes, and easy isolation from diverse environmental samples, such as sewage, sea, and dirt (15-18).
The objective of the present study was to screen and isolate lytic bacteriophages from sewage, which can be useful for the antibiotic-resistant strains of K. pneumoniae and E. aerogenes isolated from clinical specimens.
Bacterial Strains
K. pneumoniae and E. aerogenes were isolated from the clinical specimens of three hospitals and medical centers in Amol, Mazandaran, Iran during January-September 2018. Isolated bacteria were analyzed using biochemical tests according to the American Society for Microbiology standard methods to confirm the bacterial strain. Moreover, P. aeruginosa ATCC 1074, S. aureus ATCC 6538, E. coli ATCC 35218, Salmonella typhi PTCC 1639, E. aerogenes PTCC 1221, and K. pneumoniae PTCC 1290 were obtained from the microbial collection of Pasteur Institute of Iran and were used as the standard strains.
Antimicrobial Susceptibility Testing
Antibiotic susceptibility patterns were assessed by the Kirby-Bauer disk diffusion method on Mueller-Hinton agar. The susceptibility of bacteria was classified based on the Clinical and Laboratory Standards Institute guidelines (19). Twelve different kinds of antibiotics were utilized, including amikacin (30 µg), cefotaxime (30 µg), ciprofloxacin (5 µg), chloramphenicol (30 µg), gentamycin (10 µg), cefalotin (10 µg), imipenem (10 µg), nitrofurantoin (300 µg), cotrimoxazole (5 µg), tetracycline (30 µg), norfloxacin (10 µg), and nalidixic acid (30 µg) (Merck, Germany).
The strains resistant to at least three classes of antimicrobial agents were considered as MDR bacteria (19). Finally, 15 MDR-K. pneumoniae and 10 MDR-E. aerogenes were obtained from clinical isolates (Table 1). Approximately 2-3 colonies were suspended with 100 ml sterile physiological serum and were incubated at 37°C to adjust the bacterial density equal to that of 0.5 McFarland turbidity reference.
Phage Isolation and Propagation
Hospital, industrial, and urban wastewater samples were collected from a different place in Mazandaran, north of Iran during January-September 2018 (Table 2). Samples were collected in a sterile falcon and then centrifuged at 5000 g for 10 min (RS/20IV, TPMYSELKO, Japan) to remove large particulates. The supernatant was filtered using 0.22 µm syringe filters (Startech, Taiwan) and stored at 4°C. To enrich the bacteriophage (21), we added 10 ml of each sample supernatant into 100 ml exponential growth cultures (in early log phase, OD of 0.4-0.6) supplemented with 0.1 M calcium chloride (Merck, Germany). The cultures were incubated in a shaker incubator (JAL company, Iran) at 37°C and 40 rpm for 48 h. Next, the samples were centrifuged at 5000 rpm for 15 min and the supernatant was filtered through a 0.22 μM syringe filter (22).
Table 1. MDR-K. pneumonia and MDR-E. aerogenes obtained from clinical isolates
| Microorganism | Source | Sample origin | Number of samples | Abbreviation |
|---|---|---|---|---|
| K. pneumoniae | Imam Reza Hospital | Urine Urine Blood Urine Urine Chip Urine Blood Urine Wounds |
10 | Kr1 Kr2 Kr3 Kr4 Kr5 Kr6 Kr7 Kr8 Kr9 Kr10 |
| K. pneumoniae | Imam Ali Hospital | Urine Wounds Blood Urine Wounds |
5 | Ka1 Ka2 Ka3 Ka4 Ka5 |
| E. aerogenes | Imam Reza Hospital | Urine Urine Wounds Urine Chip |
5 | Er1 Er2 Er3 Er4 Er5 |
| E. aerogenes | Imam Mosabn Jafar Hospital | Urine Urine Wounds Urine Blood |
5 | Em1 Em2 Em3 Em4 Em5 |
Table 2. Place of collection of wastewater samples
| Sample number | Sampling location |
| 1 | Sewage outlet of Amirkola Children's Hospital |
| 2 | Sepidan poultry slaughterhouse wastewater outlet |
| 3 | Oil Company effluent (Mahmoud Abad) |
| 4 | Sewage of Cheshmeh Saran fish breeding pool |
| 5 | Sewage of North Pardis Recreation Complex |
| 6 | Wastewater outlet of Ghaem Hospital (Kelardasht) |
| 7 | North Hospital (Amol) |
| 8 | Imam Hossein Hospital (Neka) |
| 9 | Omidi Hospital (Behshahr) |
| 10 | Imam Hospital (Behshahr) |
| 11 | Babol Poultry Slaughterhouse |
Double-Layer Agar Technique (Plaque Assay)
We used the double-layer agar (DLA) method to examine the antibacterial ability of the isolated phages. To this end, 200 μL of bacterial culture early log phase (OD=0.2-0.4) and 200 μL of filtrates supernatant were mixed and incubated for 15 min at 37°C for proper absorption. The medium was mixed with 3 ml of LB soft agar containing 0.7% half agar (Merck, Germany) and was poured onto the bottom agar followed by swirling to produce a uniform top layer. The plates were incubated overnight at 37°C. The lysis zones or plaque formation after incubation suggested the presence of lytic phages (23).
Phage Purification
Each plaque formed on the plate surface contains approximately 105-106 phages. The established plaque was solved in 1.5 mL of LB solution. Afterwards, 200 μL of chloroform was added to eliminate bacterial contaminations according to the method mentioned in the phage isolation section. For better purification, plaque assay was performed in triplicate. The titers of each phage, isolated against MDR-bacteria from clinical samples, were represented in plaque-forming units (PFU) per mL (PFU/mL) as described by Carlson and Miller (24).
Determination of Host Range
Spot assay was applied for determining the host range of purified bacteriophage (25). Different bacteria isolated from clinical specimens and indicator bacteria strains were grown in LB broth until the early log phase (OD=0.4). Next, 300 μL of bacteria were mixed with 0.5% LB agar infused onto the underneath 1% LB agar to solidify. Afterwards, 10 μL of several dilutions of purified phage (107, 105, 103, and 101 PFU/mL) was dropped on the LB agar. The plates were incubated overnight at 37°C. The lysis zones or plaque formation were analyzed after incubation.
Phage Adsorption Assays
Diverse bacteria isolated from clinical samples were cultured overnight in LB at 37°C. Approximately 107 PFU/mL of isolated and purified phage in 500 μL was mixed with 500 μL of bacterial samples, about 108 CFU/mL. The suspension was incubated at 37°C and the medium was centrifuged at 8000 × g for 3 min after 0, 2, 4, 6, 8, 10, and 12 min. The phage titer that remained in the supernatant was examined by the DLA method. The isolated bacteria cultured in LB was exclusively utilized as a non-absorbing control.
Determine the Optimal Multiplicity of Infection
To determine the optimal multiplicity of infection (MOI) required for attaining maximum adsorption without affecting bacterial viability (26), 104 bacteria (in early log phase, OD=0.3) were cultured in a 96 well plate and mixed with several dilutions of phage (107, 106, 105, 104, 103, and 102 PFU/mL). MOIs were assessed at 2 hr, 4 hr and 8 hr after infection. The experiments were performed in triplicate. The wells containing exclusively bacteria or phage were considered as controls. The turbidity of each well was measured with a microplate enzyme-linked immunosorbent assay reader (ELX800, BioTek Instruments, USA) at 630 nm (OD=630). The best results were adopted for our phage further analysis.
One-step Growth Curve Analysis
Host bacteria were cultured in 10 ml LB broth until reaching the early log phase (OD=0.3). Afterwards, the cultured bacteria were centrifuged at 5000 × g for 10 min, the supernatant was discarded, and the pellet was resuspended (contain bacteria) in 1 mL LB broth. The phage suspension was mixed with bacteria culture medium according to MOI (Table 3) and the suspension was incubated at room temperature for 10 min for phage adsorption.
Following incubation, the non-adsorbed phage was deleted by centrifugation at 11000 × g for 30 sec and the pellet containing adsorbed phage was suspended in 10 ml of LB broth. The phage titer in the culture was determined every 10 min. The phage was purified using chloroform according to the method described in the phage isolation section (27)
Table3. General characteristics of 3 bacteriophages isolated from wastewater samples
| vB-Ea1** | vB-Kp2 | vB-Kp1* | Characteristics |
| E. aerogenes | K. pneumoniae | K. pneumoniae | Host bacteria |
| 3×1012 | 3×1011 | 15×1010 | PFU/ml |
| Clear | Clear | Clear | Plaque appearance |
| 103 | 101 | 103 | MOI |
| 10 | 10 | 20 | Latent period (min) |
| 333 | 833 | 500 | Burst size |
| Myoviridae | Myoviridae | Myoviridae | Family |
| 70 | 45 | 40 | Head diameter (nm) |
| 110 | 80 | 70 | Tail length (nm) |
| Sewage outlet of Amirkola Children's Hospital | Sepidan poultry slaughterhouse wastewater outlet | Sewage outlet of Amirkola Children's Hospital | Sampling location |
Phage Morphology Observation by Electron Microscopic
For transmission electron microscopy (TEM) observation, the sample was prepared based on Brenner and Horne protocol (27). Briefly, the purified phage suspension (109 PFU/mL) was centrifuged at 15000 × g for 150 min and the supernatant was discarded. The pellet was washed with distilled water two times. The bacteriophage was deposited on a cuprum grid with carbon-coated formvar film and was stained with 2% potassium phosphotungstate (pH: 7.2) or 1% uranyl acetate (pH: 4.5). Following air drying, the sample was observed by TEM (ZEISS EM 900, Germany) at an acceleration voltage of 50 kV.
SDS-PAGE Analysis of Phage Structural Proteins
The purified phage suspension (109 PFU/mL) was centrifuged at 8000 × g for 60 min at 4°C (Centrifuge 5424 R, Eppendorf, Germany) and the supernatant was discarded. The phage pellet was suspended in 1 mL of loading buffer containing 2.5% β-mercaptoethanol, 2% SDS, 10% glycerol, and 0.0025% bromophenol blue in 6.25 mm Tris-HCL (pH: 6.8).
The suspension was heated at 100°C for 10 min to denature the phage proteins. Next, 14 µL of denatured sample and 2 µL of protein molecular weight marker (Thermo Fisher, Germany) were loaded in 12% polyacrylamide gel (PAGE) and were electrophoresed in 100-120 MV for 5 hr. Finally, after separation, dimension gels were stained with silver nitrate (28, 29).
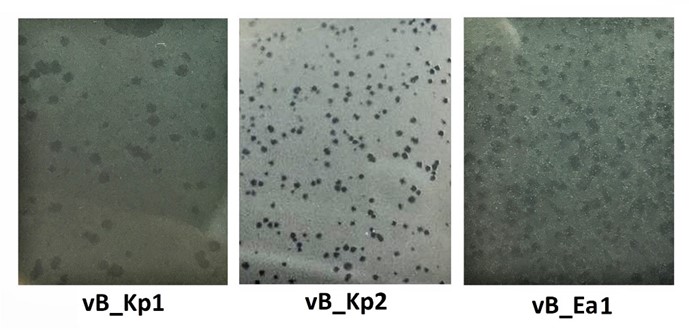
Figure 1. Primary plaques of three isolated phages
Susceptibility of Isolated Strains to the Three Purified Phages
The host range of the isolated phages (vB_Kp1, vB_Kp2, and vB_Ea1) was tested against 15 MDR-K. pneumoniae and 10 MDR-E. aerogenes clinically isolated strains and standard strains, including P. aeruginosa ATCC 1074, S. aureus ATCC 6538, E. coli ATCC 35218, S. typhi PTCC 1639, E. aerogenes PTCC 1221, and K. pneumoniae PTCC 1290. The spot assay method was employed to characterize host specificity in three different phages. Their infectivity was categorized based on plaque formation and in case the plaques were formed the strain was selected as a host of phage (Table 4).
As shown in Table 4, phages vB_Kp1 and vB_Kp2 lysed 5 (33.3%) and 7 (46.6%) samples out of 15 clinically isolated K. pneumoniae cases, respectively. Furthermore, vB_Ea1 was able to lyse 6 of 10 (60%) clinical E. aerogenes samples. The phages could not infect 11 isolated MDR-strains, including Kr1, Kr2, Kr5, Kr8, Kr9, Ka1, Ka4, Em2, Em5, Er2, and Er4. The other isolated MDR-strains were sensitive to at least one of the isolated phages. The antibiotic resistance analysis of the susceptible strains revealed that all the strains were resistant to more than three of the tested antibiotics.
Table 4. Susceptibilities of clinically isolated strains to infection with bacteriophages isolated from wastewater samples
| vB-Ea1 | vB-Kp2 | vB-Kp1 | Strain |
| - | - | - | Kr1 |
| - | - | - | Kr2 |
| - | + | + | Kr3 |
| - | + | - | Kr4 |
| - | - | - | Kr5 |
| - | + | + | Kr6 |
| - | + | - | Kr7 |
| - | - | - | Kr8 |
| - | - | - | Kr9 |
| - | + | + | Kr10 |
| - | - | - | Ka1 |
| - | + | + | Ka2 |
| - | + | - | Ka3 |
| - | - | - | Ka4 |
| - | - | + | Ka5 |
| + | - | - | Em1 |
| - | - | - | Em2 |
| + | - | - | Em3 |
| + | - | - | Em4 |
| - | - | - | Em5 |
| + | - | - | Er1 |
| - | - | - | Er2 |
| + | - | - | Er3 |
| - | - | - | Er4 |
| + | - | - | Er5 |
| + | - | - | E. aerogenes PTCC1221 |
| - | + | - | K. pneumoniae PTCC 1290 |
| - | - | - | P. aeruginosa ATCC 1074 |
| - | - | - | S. aureus ATCC 6538 |
| - | + | + | E. coli ATCC 35218 |
| - | - | - | S. typhi PTCC 1639 |
Morphology of Lytic Bacteriophages
The morphology of isolated and purified phages was analyzed using TEM (Figure 2). Phages had long tails of approximately 70, 80, and 110 nm in a non-compressed state. In various images prepared for each phage, the contracted and compressed tails could be observed. Moreover, phages vB_Kp1, vB_Kp2, and vB_Ea1 had icosahedral capsids measuring approximately 40, 45, and 70 nm, respectively. The morphological characteristics of isolated phages as long contracted tail and icosahedral capsid are similar to those of the Mayoviridae family (Table 3).
Figure 2. TEM images of isolated phages (vB_Kp1, vB_Kp2, and vB_Ea1); scale bars for each picture have been mentioned.
Optimal Multiplicity of Infection
Cultures of 104 CFU/ml host bacteria in the early log phase (OD=0.3) were infected with different amounts of designed phages and were evaluated to determine the phage titers after 2, 4, and 8 h of incubation. The optimal MOI was selected for subsequent experiments. The optimal MOIs of vB_Kp1, vB_Kp2, and vB_Ea1 were 103, 101, and 103, respectively (Table 3).
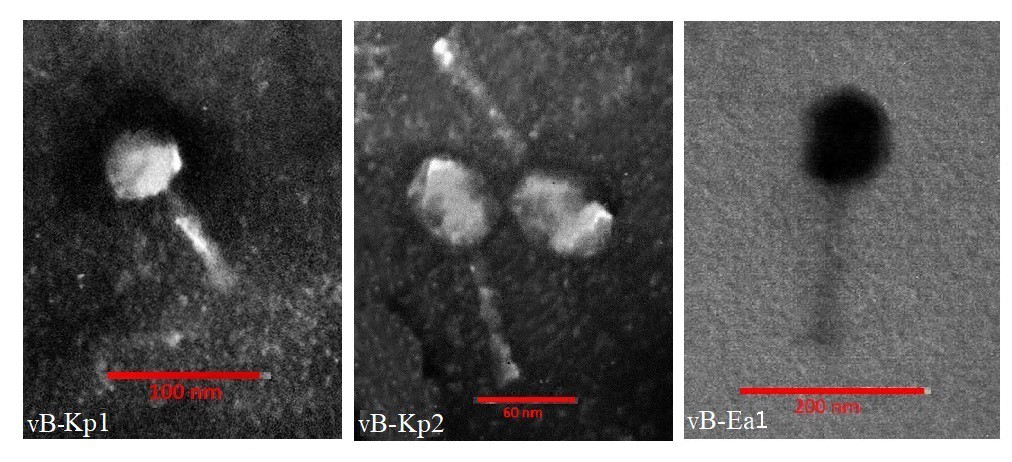
Figure 2. TEM images of isolated phages (vB_Kp1, vB_Kp2 and vB_Ea1). Scale bars for each picture have been mentioned.
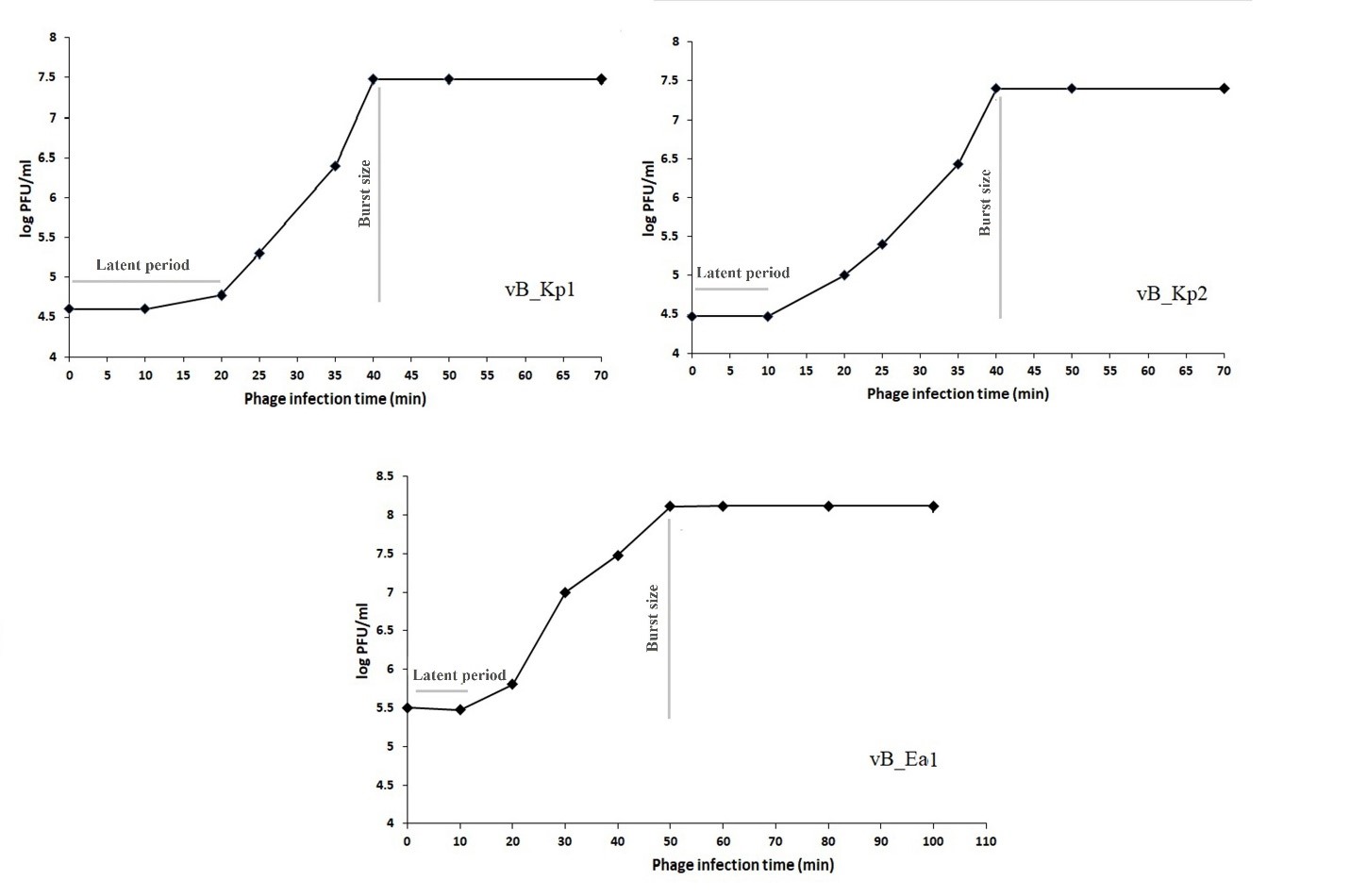
Latent Time and Isolated Phages Burst Size
Figure 3. One-step growth curve of isolated bacteriophages vB_Kp1, vB_Kp2, and vB_Ea1.
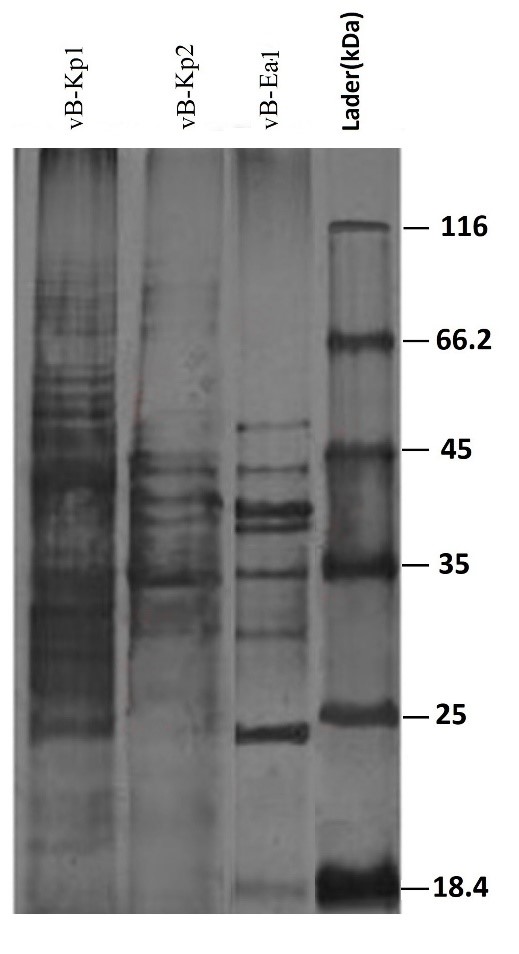
SDS-PAGE Patterns of Structural Proteins of Isolated Phages
Three isolated phages, namely vB_Kp1, vB_Kp2, and vB_Ea1 were analyzed in terms of their structural protein composition by SDS-PAGE. Many protein bands were observed in a silver-stained SDS polyacrylamide gel. All the three phages assigned to the Miroviridae family presented a different protein pattern in SDS polyacrylamide gel. Nevertheless, protein bands were observed with a size range of 18-70 kDa (Figure 4). Several bands were observed to be very strong and some were weak in SDS polyacrylamide gel.
Figure 4. SDS-PAGE patterns of structural proteins of the isolated bacteriophages vB_Kp1, vB_Kp2, and vB_Ea1
Figure 4. SDS-PAGE patterns of structural proteins of isolated bacteriophages vB_Kp1, vB_Kp2, and vB_Ea1.
The rising incidence of gram-negative antibiotic-resistant bacterial infections, such as E. aerogenes and K. pneumoniae is one of the most remarkable problems in public health elevating the associated mortality rate and financial and psychological burden on patients and the healthcare system (30-34). K. pneumoniae and E. aerogenes have been identified as potently pathogenic organisms in various infections, which show an extraordinary ability to express ESBL, KPC, and Metallo beta-lactamase making problems for successful treatment (4-7).
Therefore, for the treatment of MDR infections, efficient and affordable therapeutic approaches with fewer side effects are required. Lytic phages are similar to antibiotics in terms of significant antibacterial activity. However, therapeutic phages, at least in theory, have advantages over antibiotics. Phages have been reported to be much more effective and efficient than antibiotics in treating antibiotic-resistant infections in humans and laboratory animals.
In addition to being used to kill bacteria, phages have certain hosting properties that can be used to detect and type bacterial infections. Moreover, these products can provide an influential basis for phage therapy that requires rapid detection of bacterial targets and their sensitivity to specific phages.
Phage therapy is one of the significant, inexpensive, and specific methods that has attracted increasing attention for the treatment of MDR bacterial infections today (35, 36). However, new isolated MDR bacterial strains may be resistant to these phages. Bacteria can resist phage attack through diverse mechanisms, such as spontaneous mutations, restriction-modification systems, and adaptive immunity via the CRISPR-Cas system. Consequently, the identification of new phages and the creation of bacteriophage cocktails can be useful for improving therapeutic phages (37- 39).
Up to now, many bacteriophages have been characterized as an antibacterial agent for K. pneumoniae (40, 41) and E. aerogenes (42, 43). In the present study, first, MDR-K. pneumoniae and MDR-E. aerogenes were isolated from clinical specimens and used as the main host to screen for bacteriophages extracted from hospital wastewater. Two phages, namely vB_Kp1 and vB_Kp2 against MDR-K. pneumoniae and vB_Ea1 phage against MDR-E. aerogenes were isolated from hospital effluent.
Clear plaques are characteristic of lytic phages and all three isolated phages formed clear plaques showing their lysing potential. Electron microscopy showed that all the three phages isolated from the Myoviridae family are tail phages (44). The burst sizes of vB_Kp1, vB_Kp2, and vB_Ea1 are about 500, 833, and 333 PFU/infected cells, and the latent times are about 20, 10, and 10 min, respectively.
All three isolated bacteriophages showed several characteristics suitable for phage use, including forming clear plaques and having a short latent time and large burst size indicated by a one-step growth curve. Moreover, the SDS-PAGE patterns of structural proteins showed that all three isolated phages were similar to the protein patterns of other members of the Myoviridae family, such as PhaxI (45), ?TMA (46), AP22 (25), and LP65 (47).
Karumidze et al. isolated bacteriophages VB-KLP-5 and VB-KLOX-2 from Georgia River wastewater. Clinical samples of K. pneumoniae and K. oxytoca were used to isolate and amplify phages. Both phages had large burst sizes and were stable under different adverse conditions. The phages reported in the current study are double-stranded DNA bacterial viruses belonging to families Podoviridae and Siphoviridae. The phages were able to lyse about 63% of the Klebsiella strains, including a set of 123 clinical isolates from Georgia and the United Kingdom. The properties of these phages indicated their potential to be applied in phage therapy cocktails (40).
In 2019, Zhao et al. identified a specific lytic bacteriophage for E. aerogenes isolates called vB_EaeM_φEap-3. Based on TEM analysis, phage vB_EaeM_φEap-3 was classified as a member of the Myoviridae family. Determination of host range demonstrated that vB_EaeM_φEap-3 was able to lyse 18 of the 28 strains of E. aerogenes while showing a one-step growth curve, short latent times, and a medium burst size. Considering host range, genome, and phage parameters, vB_EaeM_φEap-3 became a suitable candidate for phage therapy programs (42).
In the present study, phages vB_Kp1 and vB_Kp2 lysed about 33.3% and 46.6% of the clinical isolates of K. pneumonia. In addition, vB_Ea1 lysed approximately 60% of E. aerogenes isolates. These percentages indicate their applicability in phage therapy. Some studies have reported a wider host range for some phages.
For example, in the study performed by Komijani et al. over two years, K. pneumoniae was isolated from 193 infected wounds in three hospitals of Isfahan that were ESBL positive. The lytic phage was isolated against K. pneumoniae and the host range, morphology, thermal stability, pH, salinity stress, and genome size were estimated. The phage was classified in the Myoviridae family due to the morphological characteristics. Out of 41 clinical specimens, 38 cases were phage sensitive (41). On the other hand, some other studies reported a very limited host range for isolated phages.
In a study by Li et al. in 2016, the phiEap-2 phage against E. aerogenes was isolated from wastewater and lysed E. aerogenes 3-sp strain. Following phage isolation, diverse properties, such as host range, phage structural proteins, genome structure and sequence, pH tolerance range, and resistance to different temperatures were investigated showing that this phage can be used for treatment (43).
Phages vB_Kp1, vB_Kp2, and vB_Ea1 could be suitable candidates for phage therapy research due to different characteristics, including short latent time, large burst size, and lyticity. However, further studies concerning resistance at different pH and temperatures, genomic sequencing, and bioinformatics studies are required.
Despite the findings of the present study, our data may not be sufficient for determining host specificity and phage type for successful therapeutic use. Therefore, further experiments, molecular analysis, complete genome sequencing, proteomics analysis, and clinical studies are necessary for selecting acceptable phage types against MDR-K. pneumoniae and MDR-E. aerogenes.
We would like to express our gratitude and thanks to all who helped during the implementation of this research. It is worth mentioning that this article is the result of a part of the dissertation of Ms. Fatemeh Habibi Nava from the Islamic Azad University, Qom Branch.
Authors declared no conflict of interests.
Received: 2020/09/16 | Accepted: 2020/11/2 | ePublished: 2021/01/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |