BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1114-en.html
2- Mashhad Branch, Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Mashhad, Iran. ,
3- Cellular and Molecular Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
4- Mashhad Branch, Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Mashhad, Iran.
Introduction
Milk contains a large number of proteins, some of which have been well characterized, such as lactoferrin, which exhibits antibacterial activity (1). Two iron-binding proteins, namely lactoferrin, and transferrin, have been shown to have biological activity in the milk of many species (2). The two proteins almost possess similar properties, such as molecular weight (80 kDa) and the ability to bind to iron (3). Lactoferrin is a monomeric glycoprotein with a high affinity to iron (4). The presence of lactoferrin has been confirmed in a large number of mammals, and the amino acid sequence of this protein has been identified in human, pig, horse, camel, cattle, buffalo, goat, and mouse (5). The third structure of lactoferrin has been characterized in the five species of human, cow, buffalo, camel, and horse, all of which have the same structures (6-10). All nutrients found in human milk are also present in camel milk. A number of investigations have recently reported the therapeutic properties of camel milk, such as anti-cancer, anti-diabetic, anti-microbial potential (11-14). Most secretions fluids, such as milk, saliva, tears, and neutrophil granules, contain this glycoprotein (15). In the immune system, lactoferrin is introduced as a part of the innate immune system because of its sensitive position on the mucosal surface as one of the agents existing at the first-line of defense to prevent the entry of microbial agents (16). Camel milk lactoferrin has been identified as a factor against Gram-positive and Gram-negative bacteria, as well as fungi and parasites (17). Proteins and peptides of the milk, such as lactoferrin, lactoperoxidase, and lysozyme, play an influential role in overall antimicrobial activity in milk (18). The milk proteins and peptides can be natural substitutes for antibiotics due to their antibacterial properties (19). Antimicrobial peptides (AMPs) are oligopeptides with varied numbers of amino acids (from 5 to more than 100) (20, 21). Some studies have analyzed the antimicrobial activity of peptides derived from lactoferrin (22-26). Camel milk lactoferrin has antibacterial activity against Escherichia coli, Salmonella typhimurium, Listeria monocytogenes, Staphylococcus aureus and Pseudomonas aeruginosa (11, 27-29). Thanks to bioinformatics tools and knowing the cleavage sites of enzymes, the identification of AMPs in natural sources, such as milk, has been feasible (30-32). Different methods are used to characterize and produce active AMPs (33). These methods are based on proteolysis using bioinform-atics software and statistical analyses to assess the role of peptides released from cleaved proteins. In this study, in order to find a novel AMP, different AMPs, derived from camel milk lactoferrin, were designed by bioinformatics approaches, and then, the most app-ropriate AMP was chosen according to the required criteria. The designed peptide was synthesized and analyzed in terms of the antibacterial activity against P. aeruginosa, A. baumannii, and S. aureus.
Materials and Methods
Bioinformatics Analysis
Selection of Effective Antimicrobial Peptide Derived from Lactoferrin
Camel milk lactoferrin is a glycoprotein that contains 708 amino acids and possesses two domains and two iron-binding sites (34). The strategy used for AMP synthesis from camel milk lactoferrin was in acco-rdance with bioinformatics approaches of a study conducted by Dziuba et al. (32). Based on the cleavage sites identified for various proteolytic enzymes, in-silico studies indicated that pepsin is capable of releasing numerous active peptides from lactoferrin (32, 35, 36). The initial structure of camel milk lactoferrin was obtained from the Uniport database with an ID of Q9TUM0. Next, pepsin was used to hydrolyze the protein, as this enzyme (pH>2) has proteolytic activity and is commonly applied to obtain bioactive peptides (37-39). Four online software tools, namely BIOPEP database, Collection of Antimicrobial Peptides Server, Antimicrobial Peptide Calculator and Predictor and Peptide cutter database were utilized to evaluate the antimicrobial properties of cleaved peptides derived from lactoferrin (40-43). The online server BIOPEP-UWM (www.uwm.edu.pl/biochemia) was utilized since it contains a library of bioactive peptides and comprises of the two essential coord-inated parts, namely grouping databases and appara-tuses for the assessment of proteins as the anteced-ents of bioactive peptides, which includes the proteol-ytic procedure plans. Another online database, CAMP (http://www.camp.bicnirrh. res.in), contains inform-ation about the conserved sequences, representing some examples and Hidden Markov Models (HMMs) of 1386 AMPs that are categorized into 45 families. On the other hand, the online database APD2 (http:-//aps.unmc.edu/AP/expectation/forecastmain.php) contains 2684 antimicrobial peptides from six kingdoms (266 from microbes, 4 from archaea, 8 from protists, 13 from parasites, 329 from plants, and 2018 from creatures) (44).
Experimental Procedures
Material
S. aureus (1074, ATCC: 6538), P. aeruginosa (1060, ATTCC: 27853), and A. baumannii (1003, ATCC: BAA-747) were purchased from the Pasteur Institute of Iran.
Peptide Synthesis
The chosen peptide was named Pepsin-Camel-Lac1 (PCL1) synthesized by MIMOTOPES Pty Ltd, Australia. In order to prepare the designed peptide, 1 mg of the designed peptide was dissolved in 1 mL of sterile water and used for the experiments.
Toxicity Assay
The Methyl thiazolyl diphenyl-tetrazolium bromide (MTT) assay was utilized to assess the toxicity of PCL1 against a human cell line. The assay is based on the decrease of the tetrazolium salt into blue formazan crystals in viable cells (45, 46). The cell line was cultured in the RPMI 1640 medium supplemented with 10% Fetal Bovine Serum (FBS), 100 ml penicillin, and 100 mg/mL streptomycin. During the cell culture period, cells were incubated at 37°C in a 95% atmosphere and 5% CO2. When the cells reached 80-90% confluence, the viability of cells was examined and passaged. In this experiment, 100 µL of the cell suspension was added to each well of a 96-well plate, each containing 100 mL of the culture medium. The cells were treated with the pre-defined concen-trations of PCL-1 and then incubated for 24 h. After dilution of PCL-1 with sterile water, cells were treated with PCL-1, and then 20 µL of the MTT solution (5 mg/mL) was added to the wells and incubated for 24 hours. After the incubation period, 50 µL of dimethyl sulfoxide (DMSO) was added to the wells to dissolve the purple formazan crystals. Afterward, according to the obtained results, the inhibitory concentration (IC50) of PCL-1 was calculated, in which 50% of the cells underwent cell death. The experiments have been carried out in triplicates, and the values were represented as the percentage of cell viability in comparison with the control cells.
Preparation of Microbial Suspension Solution
The lyophilized batches containing bacteria mentioned earlier were transferred to the liquid Mueller Hinton Broth (MHB) and incubated at 37°C for 24 h. In order to determine the antimicrobial effects, a fresh 24-hour culture medium was prepared, then 1 ml of the 24 h microbial suspension was transferred into a tube containing the MHB medium, and the turbidity of the prepared microbial suspension was assessed by McFarland standards using a spectr-ophotometer at a wavelength of 625 nm. The final suspension was used for the evaluation of drug susceptibility testing, MIC, and cell culture (47).
Determination of MIC and MBC Values by Micro-Dilution Method
The MHB medium was used to evaluate the antimicrobial effects of PCL-1 by minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) methods. In general, a sterile flat 96-well plate was used after determining the half-McFarland turbidity. In this method, 50mL of MHB medium was added to well 2 to well 11. Then, 50µL of the PCL-1 peptide was added to well 2 to well 9. Next, 50 µL of the microbial suspension was added to well 1 well 10. Afterward, the plate was incubated for 24 h. Well 1 that contains bacteria were considered as the negative control, while and well 11 containing the MHB medium was considered the positive controls. In order to assess the MBC values, the contents of the wells were cultured on the Mueller Hinton Agar medium. Lack of bacterial growth is indicative of MBC determination (47, 48).
Agar Well Diffusion Assay
The antimicrobial activity test was performed using the Agar well diffusion assay (49). This technique is broadly used to assess antimicrobial activity. In this method, the microbial suspension is distributed on the plate surface. Then, a hole is created with a diameter of 6-8 mm in sterile conditions, and then specific concentrations of the PCL-1 peptide were added to the hole and left to release into the pores of Agar. Different concentrations of PCL-1 were prepared as follows; PCL-1 was transferred into 5 sterile mi-crotubes, then 100 mL of sterile water was added into microtube 2 to 5. Next, 100 mL of PCL-1 was added to microtube 1 and was serially transferred into other microtubes until the last microtube. After that, 100µL of the contents of each microtube was isolated and added to the plate agar. After incubating the plates at 37°C for 24h, the results were examined based on the diffused area around the created hole (47).
Results
In-Silico Proteolysis of Lactoferrin
The sequence of amino acids of lactoferrin was exposed in in-silico proteolysis performed by the BIOPEP database. In-silico proteolysis can be carried out by means of some bioinformatics tools, as we employed the peptide cutter database (https://-web.expasy.org/peptide_cutter/.) (42, 50). For in-silico protein hydrolysis, a single enzyme was used and the PCL-1 peptide cleavage site was bolded (Table 1). The pepsin enzyme (pH>2) was utilized to create different peptides from lactoferrin, resulted in the production of 50 peptides with the length of 5-21 amino acids. For the statistical prediction of antimicrobial properties, the produced peptides were submitted (Table S1). A short linear peptide, PCL1, met the required criteria, as shown in Figure 1.
Table 1. Results of peptide released from BIOEP database camel milk lactoferrin.
| Enzyme |
No. of cleavages | Positions of cleavage sites |
|---|---|---|
| Pepsin (pH>2) | 188 | 2 3 5 8 9 10 11 13 14 15 16 17 18 27 40 41 62 77 78 81 82 83 84 87 90 92 100 101 110 111 122 123 124 125 127 128 130 131 138 143 144 149 150 153 155 156 157 163 170 171 188 190 191 207 209 210 211 214 215 217 226 227 233 234 237 246 247 248 265 266 285 286 287 288 289 296 297 304 305 306 307 308 316 318 319 323 324 325 326 327 336 337 338 339 342 343 349 358 359 365 366 374 375 379 380 401 402 403 404 410 411 412 413 416 417 418 419 430 441 442 451 453 467 469 470 485 486 492 494 502 504 505 506 520 522 523 542 544 545 548 549 551 560 561 567 568 578 579 582 584 585 587 588 589 590 591 592 593 608 625 626 629 630 631 636 637 647 648 650 651 657 659 660 666 667 669 670 676 678 679 684 690 691 698 700 704 705 706 |
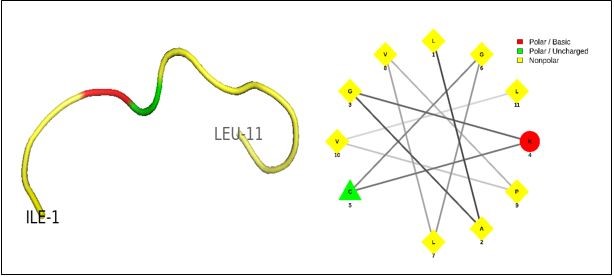
Figure 1. The PCL1 peptide picture consisted of 11 amino acids and has a liner structure.
Physicochemical Properties of AMPs
The physicochemical properties of antimicrobial peptides designed by the BIOPEP database were characterized using free web tools and the algorithms for the chosen AMP are shown (Table S1). The molecular weights of created peptides were in a range of 446 to 2493 Da (Table S1). Among 50 peptides created, 26 peptides had a pI value of less than 7, while the rest has above 7. It is now known that the molecular charge and hydrophobicity play a significant role in the functionality of AMPs (51-53). Among 50 peptides derived from lactoferrin, 14 peptides had a negative charge, 25 possess a positive charge, and 11 were neutral. In the present study, only positive AMPs were selected for further analyses. Because cationic peptides constitute a significant percentage of AMPs, as a result of their higher affinity to bind to negatively charged cell membranes of bacteria and eventually disrupt the bilayer lipid structure (54, 55). Amphipathic property is another marked parameter to choose efficient AMPs since these structures allow them to bind to hydrophilic regions; hence AMPs with both hydrophilic and hydrophobic domains have a higher priority to be used for antibacterial tests (56). The amino acid composition also plays a role in designing the antibacterial peptides. Gram-positive and Gram-negative antimicrobial peptides have a similar ratio of cysteine and lysine residues (57). As shown in Table 1, the PCL1 peptide composed of 63% hydrophobic region, 27% hydrophilic region, and 9% charged amino acids; Also, it contains 18% lysine and 9% cysteine.
Table 2. Physical and chemical characteristics of the Pepsin-Camel-Lac1 peptide.
| Position of cleavage site | Enzyme | Peptide sequence | Peptide length [aa] | Peptide mass [Da] | Seq. ID | Charge | pI |
| 430 | Pepsin (pH>2) | >30 IAGKCGLVPVL |
11 | 1069.371 | 30 | 8.22 | +1 |
Table S 1. Physical and chemical characteristics of the synthesized peptides.
| Sequence ID | Name of cleaving enzyme(s) | Resulting peptide sequence | Peptide length [aa] | Peptide mass [Da] | Position of cleavage site | pI | Charge |
|---|---|---|---|---|---|---|---|
| 1 | Pepsin (pH>2) | >1 AASKKSVRW | 9 | 1032.211 | 27 | 11.12 | +3 |
| 2 | Pepsin (pH>2) | >2 CTTSPAESSKCAQ | 13 | 1312.432 | 40 | 6.25 | 0 |
| 3 | Pepsin (pH>2) | >3 QRRMKKVRGPSVTCVKKTSRF | 21 | 2493.033 | 62 | 12.2 | +8 |
| 4 | Pepsin (pH>2) | >4 ECIQAISTEKADAVT | 15 | 1578.755 | 77 | 4.14 | -2 |
| 5 | Pepsin (pH>2) | >5 LRPIAAEV | 8 | 868.044 | 100 | 6.25 | 0 |
| 6 | Pepsin (pH>2) | >6 GTENNPQTH | 9 | 996.989 | 110 | 5.5 | -1 |
| 7 | Pepsin (pH>2) | >7 YAVAIAKKGTN | 11 | 1135.329 | 122 | 9.72 | +2 |
| 8 | Pepsin (pH>2) | >8 KSCHTGL | 7 | 744.864 | 138 | 8.22 | +1 |
| 9 | Pepsin (pH>2) | >9 GRSAG | 5 | 446.464 | 143 | 10 | +1 |
| 10 | Pepsin (pH>2) | >10 NIPMG | 5 | 530.640 | 149 | 5.5 | 0 |
| 11 | Pepsin (pH>2) | >11 TGPPEP | 6 | 596.638 | 163 | 3.81 | -1 |
| 12 | Pepsin (pH>2) | >12 LQKAVAK | 7 | 756.944 | 170 | 10 | +2 |
| 13 | Pepsin (pH>2) | >13 FSASCVPCVDGKEYPNL | 17 | 1829.073 | 188 | 4.47 | -1 |
| 14 | Pepsin (pH>2) | >14 CAGTGENKCACSSQEP | 16 | 1584.711 | 207 | 10 | -1 |
| 15 | Pepsin (pH>2) | >15 LQDGAGDVA | 9 | 844.877 | 226 | 3.53 | -2 |
| 16 | Pepsin (pH>2) | >16 VKDSTV | 6 | 647.726 | 233 | 6.25 | 0 |
| 17 | Pepsin (pH>2) | >17 PAKADRDQY | 9 | 1063.135 | 246 | 6.25 | 0 |
| 18 | Pepsin (pH>2) | >18 LCPNNTRKPVDASQECH | 17 | 1912.125 | 265 | 7.09 | 0 |
| 19 | Pepsin (pH>2) | >19 ARVPSHAVVARSVNGKEDL | 19 | 2005.265 | 285 | 9.06 | +1 |
| 20 | Pepsin (pH>2) | >20 LVKAQEK | 7 | 814.980 | 296 | 8.88 | +1 |
| 21 | Pepsin (pH>2) | >21 GRGKPSA | 7 | 671.754 | 304 | 11.12 | +2 |
| 22 | Pepsin (pH>2) | >22 GSPAGQKD | 8 | 758.786 | 316 | 6.25 | 0 |
| 23 | Pepsin (pH>2) | >23 RIPSKIDSG | 9 | 972.109 | 336 | 8.88 | +1 |
| 24 | Pepsin (pH>2) | >24 ITAIRG | 6 | 629.757 | 349 | 10 | +1 |
| 25 | Pepsin (pH>2) | >25 LRETAAEVE | 9 | 1017.104 | 358 | 4.14 | -2 |
| 26 | Pepsin (pH>2) | >26 RRAQVV | 6 | 727.865 | 365 | 11.97 | +2 |
| 27 | Pepsin (pH>2) | >27 RRAQVV | 8 | 807.830 | 374 | 11.97 | +2 |
| 28 | Pepsin (pH>2) | >28 SRQSNQSVVCATASTTEDCIA | 21 | 2171.339 | 401 | 4.47 | -1 |
| 29 | Pepsin (pH>2) | >29 KGEADA | 6 | 589.603 | 410 | 4.47 | -1 |
| 30 | Pepsin (pH>2) | >30 IAGKCGLVPVL | 11 | 1069.371 | 430 | 8.22 | +1 |
| 31 | Pepsin (pH>2) | >31 AESQQSPESSG | 11 | 1106.068 | 441 | 3.53 | -2 |
| 32 | Pepsin (pH>2) | >32 DCVHRPVKG | 9 | 1010.179 | 451 | 8.22 | +1 |
| 33 | Pepsin (pH>2) | >33 AVAVVRKANDKITW | 14 | 1570.855 | 467 | 10 | +2 |
| 34 | Pepsin (pH>2) | >34 RGKKSCHTAVDRTAG | 15 | 1586.789 | 485 | 10 | +3 |
| 35 | Pepsin (pH>2) | >35 NIPMGP | 6 | 627.756 | 492 | 5.5 | 0 |
| 36 | Pepsin (pH>2) | >36 KNTDSCRF | 8 | 970.068 | 502 | 8.22 | +1 |
| 37 | Pepsin (pH>2) | >37 SQSCAPGSDPRSKL | 14 | 1432.571 | 520 | 8.22 | +1 |
| 38 | Pepsin (pH>2) | >38 CAGNEEGQNKCVPNSSERY | 19 | 2085.207 | 542 | 4.7 | -1 |
| 39 | Pepsin (pH>2) | >39 LAENVGDVA | 9 | 886.957 | 560 | 3.53 | -2 |
| 40 | Pepsin (pH>2) | >40 VKDVTV | 6 | 659.781 | 567 | 6.25 | 0 |
| 41 | Pepsin (pH>2) | >41 DNTDGKNTEQ | 10 | 1121.082 | 578 | 4.14 | -2 |
| 42 | Pepsin (pH>2) | >42 NGTRKPVTEAESCHL | 15 | 1641.819 | 608 | 7.19 | 0 |
| 43 | Pepsin (pH>2) | >43 PVAPNHAVVSRIDKVAH | 17 | 1810.089 | 625 | 9.06 | +1 |
| 44 | Pepsin (pH>2) | >44 GRNGQDCPGK | 10 | 1031.111 | 647 | 8.22 | +1 |
| 45 | Pepsin (pH>2) | >45 QSKTKN | 6 | 704.781 | 657 | 10 | +2 |
| 46 | Pepsin (pH>2) | >46 NDNTEC | 6 | 694.671 | 666 | 3.53 | -2 |
| 47 | Pepsin (pH>2) | >47 QGKTTY | 6 | 696.758 | 676 | 8.88 | +1 |
| 48 | Pepsin (pH>2) | >48 LGPQY | 5 | 576.650 | 684 | 5.5 | 0 |
| 49 | Pepsin (pH>2) | >49 VTAIAK | 6 | 601.744 | 690 | 8.88 | +1 |
| 50 | Pepsin (pH>2) | >50 RRCSTSP | 7 | 805.907 | 698 | 10 | +2 |
Table S 2. Predict Antimicrobial Peptides.
| Results with Support Vector Machine (SVM) classifier | Results with Random Forest Classifier | Results with Artificial Neural Network (ANN) classifier | Results with Discriminant Analysis classifier | |||||
|---|---|---|---|---|---|---|---|---|
| Seq. ID. | Class | AMP Probability | Class | AMP Probability | Class | Class | AMP Probability | |
| 1 | AMP | 0.941 | AMP | 0.584 | AMP | NAMP | 0.094 | |
| 2 | NAMP | 0.016 | NAMP | 0.3475 | NAMP | NAMP | 0.029 | |
| 3 | NAMP | 0.038 | AMP | 0.6 | NAMP | NAMP | 0.421 | |
| 4 | NAMP | 0.095 | NAMP | 0.2235 | NAMP | NAMP | 0.004 | |
| 5 | NAMP | 0.004 | NAMP | 0.331 | NAMP | NAMP | 0.003 | |
| 6 | NAMP | 0.138 | AMP | 0.603 | NAMP | NAMP | 0.004 | |
| 7 | AMP | 0.595 | NAMP | 0.215 | AMP | AMP | 0.522 | |
| 8 | AMP | 0.714 | NAMP | 0.332 | NAMP | NAMP | 0.024 | |
| 9 | NAMP | 0.000 | NAMP | 0.289 | NAMP | NAMP | 0.000 | |
| 10 | NAMP | 0.002 | NAMP | 0.347 | AMP | NAMP | 0.001 | |
| 11 | AMP | 0.996 | AMP | 0.691 | NAMP | NAMP | 0.000 | |
| 12 | NAMP | 0.239 | NAMP | 0.374 | AMP | NAMP | 0.147 | |
| 13 | NAMP | 0.144 | NAMP | 0.145 | NAMP | NAMP | 0.033 | |
| 14 | NAMP | 0.230 | NAMP | 0.298 | NAMP | NAMP | 0.056 | |
| 15 | AMP | 0.929 | NAMP | 0.354 | NAMP | NAMP | 0.001 | |
| 16 | NAMP | 0.008 | NAMP | 0.347 | NAMP | NAMP | 0.000 | |
| 17 | NAMP | 0.011 | AMP | 0.5275 | NAMP | NAMP | 0.001 | |
| 18 | NAMP | 0.063 | NAMP | 0.083 | NAMP | NAMP | 0.004 | |
| 19 | NAMP | 0.345 | NAMP | 0.17 | NAMP | NAMP | 0.036 | |
| 20 | NAMP | 0.002 | NAMP | 0.297 | NAMP | NAMP | 0.004 | |
| 21 | NAMP | 0.001 | NAMP | 0.3515 | NAMP | NAMP | 0.001 | |
| 22 | NAMP | 0.000 | NAMP | 0.3385 | NAMP | NAMP | 0.000 | |
| 23 | NAMP | 0.093 | NAMP | 0.265 | NAMP | NAMP | 0.009 | |
| 24 | NAMP | 0.000 | NAMP | 0.4965 | AMP | NAMP | 0.263 | |
| 25 | NAMP | 0.001 | NAMP | 0.2205 | NAMP | NAMP | 0.000 | |
| 26 | NAMP | 0.008 | NAMP | 0.455 | NAMP | NAMP | 0.019 | |
| 27 | NAMP | 0.025 | NAMP | 0.378 | NAMP | NAMP | 0.000 | |
| 28 | NAMP | 0.033 | NAMP | 0.1255 | NAMP | NAMP | 0.002 | |
| 29 | NAMP | 0.216 | NAMP | 0.455 | NAMP | NAMP | 0.000 | |
| 30 | NAMP | 0.146 | AMP | 0.5805 | AMP | AMP | 0.949 | |
| 31 | NAMP | 0.387 | NAMP | 0.364 | NAMP | NAMP | 0.000 | |
| 32 | NAMP | 0.003 | NAMP | 0.3025 | AMP | NAMP | 0.000 | |
| 33 | NAMP | 0.399 | AMP | 0.567 | AMP | NAMP | 0.326 | |
| 34 | NAMP | 0.188 | NAMP | 0.186 | NAMP | NAMP | 0.003 | |
| 35 | NAMP | 0.005 | NAMP | 0.4345 | AMP | NAMP | 0.003 | |
| 36 | NAMP | 0.004 | NAMP | 0.3385 | NAMP | NAMP | 0.187 | |
| 37 | NAMP | 0.008 | NAMP | 0.1685 | NAMP | NAMP | 0.003 | |
| 38 | NAMP | 0.178 | NAMP | 0.2445 | NAMP | NAMP | 0.039 | |
| 39 | AMP | 0.593 | NAMP | 0.3495 | NAMP | NAMP | 0.001 | |
| 40 | NAMP | 0.007 | NAMP | 0.443 | NAMP | NAMP | 0.000 | |
| 41 | NAMP | 0.026 | AMP | 0.627 | NAMP | NAMP | 0.000 | |
| 42 | NAMP | 0.060 | NAMP | 0.0225 | NAMP | NAMP | 0.001 | |
| 43 | NAMP | 0.256 | NAMP | 0.0655 | NAMP | NAMP | 0.014 | |
| 44 | NAMP | 0.002 | NAMP | 0.2105 | NAMP | NAMP | 0.001 | |
| 45 | AMP | 0.999 | AMP | 0.501 | AMP | NAMP | 0.063 | |
| 46 | AMP | 0.984 | NAMP | 0.336 | NAMP | NAMP | 0.017 | |
| 47 | AMP | 0.760 | NAMP | 0.3345 | NAMP | NAMP | 0.002 | |
| 48 | NAMP | 0.052 | NAMP | 0.294 | AMP | NAMP | 0.041 | |
| 49 | NAMP | 0.000 | AMP | 0.503 | AMP | NAMP | 0.169 | |
| 50 | AMP | 0.999 | NAMP | 0.4025 | NAMP | NAMP | 0.086 | |
Prediction of the Antimicrobial Activity of Produced Peptides During In-Silico Proteolysis from Lactoferrin
The in-silico proteolysis of lactoferrin leads to the generation of short peptides. Among the created peptides, the PCL-1 peptide shows anti-bacterial, anti-viral, and anti-tumor activity due to its high positive charge and ability to interact with negatively charged substrates, such as glycosaminoglycan, lipopolys-accharide, heparan, phosphatidylserine, and nucleic acids (58, 59). The design of peptides varies depending on the biological activity of these macromolecules (22). Many peptides have a wide range of activity, such as the antibacterial and anti-cancer properties (60). For example, the design of AMPs derived from the lactoferrin protein has been conducted using the CAMP tools. To this aim, Four software tools have been employed, namely Random Forest, Support Vector Machines, Artificial Neural Network, and Discriminant Analysis (41). In this analysis, the resultant score was in excess of 0.45; therefore, the PCL1 peptide was listed as AMPs, and a positive classification was obtained for at least two statistical methods (Table 3).
Table 3. The characteristics of PCL1 peptide released during in-silico proteolysis and prediction.
| SVM classifier | RF Classifier | ANN classifier | DA classifier | ||||
| Sequence | Class | AMP Probability | Class | AMP Probability | Class | Class | AMP Probability |
| IAGKCGLVPVL | NAMP | 0.146 | AMP | 0.5805 | AMP | AMP | 0.949 |
Four statistical models in the CAMP database: SVM, RF, ANN and DA classiers that used for proteolysis and prediction peptides
Toxicity Assay
In order to achieve a linear regression equation of the concentrations of PCL-1 versus the growth inhibition, Microsoft Excel software was used, and the values were expressed as the means and standard deviation of the means of three separate experiments. The below equation was used to measure the growth curve and draw a plot:
Growth inhibition= (control OD-sample OD)/control OD*100 (61)
Finally, the IC50 values of specimens, representing a concentration of the peptide by which 50% of bacteria are eliminated, were obtained from the growth curve. The SPSS software version 16 was used for the analysis of the obtained data, and the difference between various treatments was analyzed by one-way analysis of variance (one-way ANOVA) followed by Tukey՛s post hoc test (Table 4). The MTT results showed that PCL-1 has no significant effect on the viability of the cell line used (Figure 2).

Figure 2. MTT cell line with PCL1 peptide with sig= 0.00
Table 4. Results of analysis using SPSS technology of mixed PCL1 peptide levels on cell lines.
| Sum of Squares | df | Mean Square | F | Sig | |
| Between Groups | 1.500 | 5 | 0.3 | 8.498 | 0.001 |
| Within Groups | 0.424 | 12 | 0.035 | ||
| Total | 1.924 | 17 |
Determination of MIC and MBC Values by Micro-Dilution Method
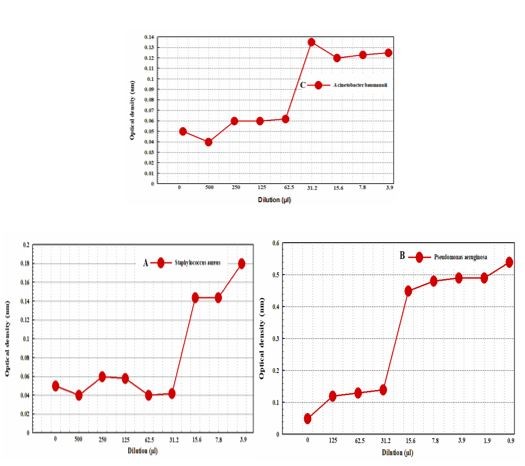
The MIC values of bacterial strains were calculated by the micro-dilution method. After two hours of incubation, the turbidity of samples was read at wavelengths of 490 and 630nm using an ELISA reader. The well in which bacterial growth inhibition occurred was regarded as the MIC values and used to determine the MBC value. Following the incubation period, the first dilution in which bacteria did not grow on the agar plate was regarded as the MBC value. According to Figure 3, the MIC values for S. aureus in response to PCL-1 was reported to be 31.25µg/mL, while the MBC value was 500µg/mL (Figure 3A).
The MIC and MBC values for P. aeruginosa were 31.25 µg/mL and 125 µg/mL, respectively (Figure 3B). In addition, the values of MIC and MBC for A. baumannii were 62.5µg/mL and 500µg/mL, respectively (Figure 3C).
Figure 3. MIC and MBC analyze outcomes for the three bacterial strains that were tested in A, B, and C for S.Aureus, P.Aeruginosa, and A.Baumannii.
Agar Well Diffusion Assay
The diameter created around the holes in agar indicates the effect of PCL-1 on the inhibition growth of bacterial strains. The results demonstrated that PCL-1 had no significant effect on the growth inhibition of the three bacterial strains when compared with the control group (Figure 4).
Figure 4. Results of agar well diffusion assay for Staphylococcus Aureus, Pseudomonas Aeruginosa, and Acinetobacter Baumannii are respectively in A, B, and C.
Discussion
In an in-silico proteolysis conducted by Dziuba et al., 11 new peptides with potential antimicrobial activity were picked from all the digested peptides in the milk protein (32). Jahani et al. demonstrated that lact-oferrin to be effective as an antibacterial against Gram-positive (Staphylococcus epidermidis, Bacillus cereus) and Gram-negative (Campylobacter jejuni, Salmonella spp.) bacteria. However, it was more effective on the Gram-positive instead of the Gram-negative bacteria (62). In line with the investigation of peptides derived from milk lactoferrin through the milk fermentation and assessment of their anti-bacterial effects, Korheonan et al. reported that milk proteins contain a rich source of peptides that could be potentially released and activated. For example, when these peptides are activated during gastric-intestinal digestion or milk fermentation, they could be involved in modulatory processes in living systems (33). Bushra Niaz et al. reported that due to the association of lactoferrin with enterobacterial lipopolysaccharide (LPS), it acts as a permeabilizing agent of Gram-negative bacteria and destabilizes the bacterial membrane and thus enhance bacterial permeability. Lactoferrin is not an effective anti-bacterial agent of its own, but allows those peptides that have been derived from it, whose hydrophobicity impose a limit on their effectiveness, to enter the bacterial membrane as an antibacterial agent.(63). As a result of direct interaction between protein or lactoferrin-derived peptides, Farnaud and Evans identified that it possesses bactericidal activities (25). Cameleers believe that camel milk is more resistant to spoilage than the milk of other animals, and it has a longer shelf life. The effect of lactoperoxidase can be one of the significant factors leading to camel milk resistance to spoilage. In this work, antimicrobial peptides were identified from camel milk lactoferrin using bioinformatics; then, the PCL-1 peptide was selected, and the activity of this peptide was evaluated in-vitro. In the first step, the hydrolysis of lactoferrin was performed using the pepsin enzyme using the BIOPEP database by which 50 peptides were produced containing 5–21 amino acids. The physicochemical properties of the synthesized peptides were investigated using online tools, such as the APD2 and Peptide cutter databases, to select appropriate AMPs. The general properties of the generated peptides, including molecular weight, charge, and PI values, were analyzed to select the appropriate antimicrobial peptides (Table S1). Among the created peptides, two peptides met the initial criteria, but since the presence of lysine and cysteine amino acids in AMPs is an advantage, the peptide with an ID of 30 (PCL-1 peptide) was selected for further analyses (Table 1). Four tools of the CAMP database (ANN, DA, RF, SVM) were employed to determine the antibacterial activity of the created peptides in this study. The PCL-1 peptide was classified as AMPs since it had parameters with scores of higher than 0.45, including RF: 0.5805 and DA: 0.949 (Table 3). After synthesizing the PCL-1 peptide, the viability of bacteria in response to PCL-1, MBC, MIC, agar well diffusion was assessed in order to determine the antimicrobial activity, as well as toxicity against the human cell line. The MTT assay showed that this peptide had no toxicity against the human cell line. Our findings also showed the MIC and MBC values for S. aureus, P. aeruginosa, and A. baumannii were 31.25/500, 21.25/125, and 62.5/500, respectively. The agar well diffusion assay showed that the analyzed bacteria were resistant to the PCL-1 peptide. The experimental results of this study showed that the PCL-1 peptide had no antimicrobial properties.
Conclusion
There are naturally occurring bioactive peptides in fermented dairy products, such as yogurt, butter, milk, and cheese, which are inactivated in primary sources, and they could be activated by digestion of milk, milk fermentation, exposure to proteolytic starter media, or hydrolysis using proteolytic enzymes. Milk-derived peptides exhibit super-beneficial activity, even through oral consumption. In this study, pepsin-digested peptides in milk lactoferrin were identified by bioinformatics tools. After the synthesis of PCL-1, the MTT assay was applied to assess the toxicity of the selected peptide against the human cell line. The results showed that the chosen peptide had no toxicity against the human cell line or bacterial strains.
Abbreviations
Antimicrobial peptides: AMPs, Collection of Antimicrobial Peptides: CAMP, Antimicrobial Peptide Calculator and Predictor: APD2, Pepsin-Camel-Lac1: PCL1, Methyl thiazolyl diphenyl-tetrazolium bromide: MTT, Mueller Hinton Broth: MHB, Minimum inhibitory concentration: MIC, Minimum bactericidal concen-tration: MBC, Agar well diffusion assay: AWDA, Random Forest: RF, Support Vector Machines: SVM, Artificial Neural Network: ANN, Discriminant Analysis: DA.
Acknowledgements
I want to thank all those who have supported me in this work. It is worth mentioning that this essay is a thesis by Ms. Elnaz 's study at Islamic Azad University, branch of Sabzevar, Iran.
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
All authors gave their consent for publication.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2020/04/25 | Accepted: 2021/02/24 | ePublished: 2021/06/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |