BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1048-en.html
2- Department of Parasitology and Mycology, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- Department of Microbiology and Immunology, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran, IRAN ,
.
Toxoplasma gondii is a mandatory intracellular parasite that causes toxoplasmosis. About 500 million to one billion people in the world are infected with the parasite, which is mainly caused by eating undercooked meat, fruits, vegetables, or being exposed to soil and water contaminated with the parasite's infected eggs. (1). Manifestations of the disease range from mild flu-like symptoms to lymphadenopathy and Chorioretinitis. Transmission of a primary (acute) infection from a pregnant mother to the fetus is largely asymptomatic and can lead to miscarriage or congenital abnormalities such as hydrocephalus, microcephaly, intracranial calcification, Chorioretinitis, blindness, epilepsy, mental retardation, or problems in motor abilities (2). On the other hand, the activation of a chronic infection in the nervous system following a weakened or impaired immune system in people with AIDS, organ transplants, or in patients with lymphoproliferative diseases can lead to central nervous system damage or complications such as encephalitis for which lack of in time diagnosis and proper treatment can have deadly consequences (3). Early detection of acute and chronic toxoplasmosis, followed by appropriate drug treatment in at-risk individuals, can reduce the severity of symptoms and the occurrence of life-threatening injuries (4). Therefore, the aim of this study was to use recombinant gra7 protein to design immunochromatographic methods for rapid diagnosis of specific IgG against T. gondii in three minutes.
Toxoplasma Culture and DNA Extraction
This experimental study was performed in 2017 with 204 serum samples from different laboratories in Tehran using non-probable sampling method. Toxoplasma-induced RH angles were used in frozen form from the Quality Control Department of the Razi Vaccine and Serum Research Institute. To remove the preservative, the parasite was washed once with PBS solution and then cultured twice by successive intracranial passages in the mouse. For the reproduction and maintenance of the parasite, 0.5 mL of peritoneal fluid containing 2×105 live parasites was injected into each mouse and 100 µL / mL of penicillin was injected intraperitoneally. After 3 to 4 days, the peritoneal cavity of infected mouse was washed with 5 mL of cold PBS buffer, and the tacos were collected and stored at -20˚C. To extract the genomic DNA, the toxoid plasma toxins were extracted from the DNA extraction kit by Synagen (Iran) in the DNG method according to the manufacturer's instructions.
Primer Design for GRA7 Gene and PCR
The gra7 gene sequence was extracted from the NCBI gene bank and designed with the enzyme sites of BglII and XhoI. The sequence of primers was confirmed using Gene Runner software.
GRA7 Forward: CAGCCCAGATCTGATGGCACGACACGCAAT
GRA7 Reverse: GTGGTGCTCGAGTTACTGGCGGGCATCCTC
The gra7 gene was amplified using PCR. The gra7 gene proliferation timing program using PCR includes initial denaturation for 5 minutes and 96°C, secondary denaturation for 30 seconds and 95°C temperature, Annealing for 30 seconds and 58°C temperature, Extension for 1 minute and temperature 7 C72 and final extension for 10 minutes at 72°C. In this process, 0.4 µM of each primer, 200 µm of any dNTP type, 1.5 unit / mL of Taq polymerase enzyme, 5 µL PCR buffer containing MgSO4 and 200 ng of the sample DNA with a final volume of 50 μL were used.
Preparation of Recombinant Plasmid and Cloning
The plasmid pET-32a (+) vector was first cut using BglII and XhoI and added to the purified PCR product. By adding the enzyme T4 ligase, the gene was added to the Recombinant Plasmid.
The recombinant plasmid was transferred to the Competent Cell Escherichia coli DH5α by heat shock. The bacterium was cultured in an LB culture medium containing 100 µg / mL of antibiotic. The recombinant plasmid was extracted using double enzyme digestion on gra7 -pET-32a (+) and PCR for the gra7 gene to confirm the accuracy of bacterial transformation.
Expression, Confirmation and Purification of GRA7 Protein
The multiplied recombinant plasmid was transferred to E. coli Rosetta (DE3). A colony was removed from the newly transformed plate and cultured in a tube containing liquid LB and ampicillin, and the next day in a 25 mL Erlenmeyer flask. Four, six, and eight hours after induction, the environment was sampled. The collected samples were centrifuged at 6000 rpm and the final precipitate was stored in a freezer at -20°C. The Western blot method was used to confirm the gene expression. In order to purify gra7 by Ni-NTA method, first the cell sediment was melted at room temperature and the cells were lysed and then the cellular lyses were evaluated directly by SDS-PAGE. Also, the lyses-resin mixture was carefully passed through the Ni-NTA chromatography column and its output was collected.
Blotting Test
At this stage, the antigen gra7 was cut on the nitrocellulose paper and after the blocking phase with bovine serum albumin, the serum of patients with toxoplasmosis was evaluated with 1:30 dilutions. In this study, 204 serums from different laboratories that were examined and collected by CLIA method were used. There were 30 serums for people with clinical symptoms, 70 serums with IgM antibodies to Toxoplasma, 74 serums with IgG antibodies against Toxoplasma and 30 negative serums (no IgG antibodies against Toxoplasma). Also, 30 serum samples of people with IgG antibodies against other diseases, all of which were negative for IgM antibodies and IgG antibodies to toxoplasma, were evaluated for Bovine Serum Albumin (BSA). Ventricular leishmaniasis (n = 5), Strongyloidiasis (n = 1), malaria (n = 13), fascioliasis (n = 4), hepatitis (n = 3), hydatid cyst (n = 4) were used.
Immunochromatography Strip
After preparing the colloidal gold and conjugating the Anti-human IgG according to the relevant protocol (18), the tape was designed and tested. The conjugated solution was poured on the conjugation pad. A recombinant gra7 antigen suspension was added to the test line area. The control line was sampled with antibodies against the mouse antibody. All sheets were cut to a width of 4 mm.
The accelerated method was used to evaluate the kit stability time. The shelf life of the kit was calculated for 24 months.
After DNA extraction from the parasite, the PCR reaction was performed using specific primers on the gene gra7. The gene proliferation band was 726 base pair (Figure 1).
.png)
Figure 1. Electrophoresis of gra7 gene on 1% agarose gel. Column 1: Marker 100 bp, Column 2: Multiplied part of the gra7 gene
The purified PCR product as well as the purified pET-32a (+) plasmid were cut with BglII and XhoI cutting enzymes and then connected to each other with the same end, and the recombinant pET-32a (+) -GRA7 plasmid was produced. The recombinant plasmid was then transferred to the bacterium and cultured in the presence of the antibiotic ampicillin. At this stage, the initial recombinant colonies were confirmed by specific primers (Figure 2 a, b).
.png)
Figure 2. Confirmation of bacterial transformation with recombinant plasmid. a) Quick check test to confirm the presence of recombinant plasmid in bacteria. b) Colony PCR test to confirm gra7 gene in vector.
DNA was extracted from confirmed colonies and finalized by double enzymatic digestion (Figure 3).
Protein expression was induced in IPTG-stimulated bacteria and then confirmed by SDS-PAGE (Figure 4a). The dot blot test was performed using human serum with IgG antibody against Toxoplasma.
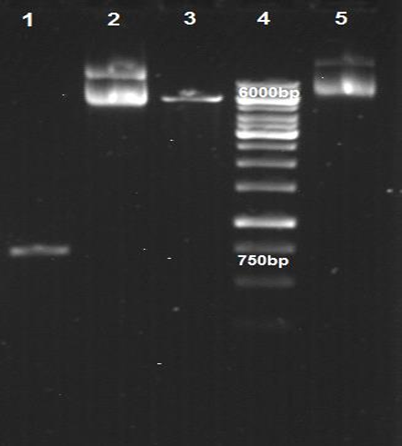
Figure 3. Digestive digestion of pET-32a (+) with XhoI and BglII enzymes and PCR product of G7 toxin gene. Column 1: gra7 gene, Column 2: Plasmid without gra7, Column 3: Plasmid after enzymatic digestion, Column 4: Molecular size index 1 kbp, Column 5: recombinant plasmid plas-32a (+) - gra7

Figure 4. Electrophoresis of lyse bacterial cell with pET-32a (+)-gra7 plasmid and evaluation of recombinant protein expression r gra7 on SDS-PAGE gel. a) Column 1: pET-32a vector (+), column 2: molecular weight index, column 3: pET-32a-gra7 four hours after induction, column 4: pET-32a-gra7 six hours after induction, column 5 : pET-32a-gra7 Eight hours after induction, column 6: recombinant purified protein r gra7. b) Western blot protein gra7 recombinant using conjugated Rabbit anti human IgG. Column 1: Protein marker, Column 2: recombinant GRA7 protein.
Strip Test
The strip test in the control line section showed a significant red color. However such a significant color was not observed in the test line section with dilutions higher than 1: 8 (Figure b5). Therefore, to perform this test, all serum samples were diluted with a 1: 8 dilution with a buffer. The sensitivity and specificity of the strip test were 100% and 96.7%, respectively.

Figure 5. Strip test for rapid detection of Toxoplasma anti-gra7 antibody. a) Schematic image of preparation of strip test components. b) Initial evaluation with the help of positive serum samples with different dilutions
Stable Kit Strip
Using the accelerated method, the stabilization time of the kit at 37°C was set at 16 months. Using conventional formulas, it is approximately equivalent to 32 months of stability at 4°C.
Most diagnostic tests used are immunological methods of antibody tracking, each of which has its drawbacks. On the other hand, identifying the specific antigen of the acute phase of the disease is a key step in designing diagnostic methods. Some studies have used the potential of GRA7 protein to diagnose with the ELISA test and reported an 80% sensitivity and 90% specificity for the test (11). For an accurate, quick and in time diagnosis, the present study, aimed at using gra7 antigen to design a rapid diagnostic test for toxoplasmosis for the first time, by immunocromatography to eliminate the disadvantages of conventional identification methods as much as possible.
Another group of researchers used SAG2 and ROP2 recombinant antigens to diagnose gonadal toxoplasmosis infection. The suggestion of using the above-mentioned recombinant antigens to make vaccines was emphasized (28). The French researchers also designed the IgG and IgM toxoplasma antibody detection strip in patients' serum and compared it with Abbott's CLIA Automatic Architect method, and the sensitivity and specificity were 97% and 96%, respectively. Another group of researchers designed a dedicated IgG strip test against toxoplasma using a recombinant SAG1 antigen that could replace the ELISA method with natural antigens. This number test helped to identify acute phase patients, and therefore suggested that it be used alongside ELISA for further study at the national level (31).
In 2019, another study aimed to develop a simple, portable, and rapid method for detecting toxoplasmosis serum based on the recombinant protein of T. gondii SAG1 (rSAG1) and GRA7 (rGRA7). It was found that IgM rGRA7-Dot-ELISA sensitivity and specificity were 87.5% and 91.1%, respectively (32).
In 2020, a study was performed in Japan to diagnose immunocompromised Gondi antibodies in cats by immunochromatographic imaging based on gra7 antigen. The results of this study showed that TgGRA7-ICT is a reliable test for anti-T diagnosis (33).
Using a test designed by the French company LDBIO, a group of American scientists tested the IgG and IgM antibodies in serum by simply examining them with 100% sensitivity and specificity.
In this study, for the first time, a strip test was designed using a recombinant gra7 antigen to diagnose toxoplasmosis.
This study, by selecting the appropriate antigen based on the important cellular and clinical characteristics of the parasite and using the results of previous tests, led to the successful development and evaluation of the rapid diagnosis of toxoplasmosis. Therefore, the results of this study can reduce the detection time by providing a quick screening solution for people suspected of having toxoplasmosis and also make it easier for a wide range of people to interpret the test results.
In this study, selecting the appropriate antigen based on the important cellular and clinical characteristics of the parasite along with the use of the results of previous tests led to the successful construction and evaluation of the rapid diagnosis of toxoplasmosis. Therefore, the results of this study can reduce the detection time by providing a quick screening solution for people suspected of having toxoplasmosis and also make it easier for a wide range of people to interpret the test results.
This work has been supported by Shahid Beheshti University of Medical Sciences with the number 265. The authors thank the Vice Chancellor for Research of Shahid Beheshti University of Medical Sciences.
Authors declared no conflict of interests.
Received: 2019/11/27 | Accepted: 2019/12/29 | ePublished: 2020/01/1
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







