BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1001-en.html
2- Department of Microbiology and Microbial Biotechnology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran
3- Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Khorramabad, Iran
.
L-asparaginase is a well-known enzyme acting on neoplastic cell diseases, especially acute lymphoblastic leukemia (ALL), therefore, it can inhibit their growth in the blood circulatory system (1) L-asparaginase activity leads to hydrolysis of L-asparagine into aspartic acid and ammonium. This enzyme plays a crucial role as a promising agent in the current treatment methods of cancer therapy, through removing asparagines that thus, could cause to inhibit the growth of tumor cells (2,3) L-asparaginase is used as an efficient antitumor drug and has been obtained either from Escherichia coli or Erwinia caratovora (4,5). Therefore, L asparaginase originated from bacterial species had been considered as the main source for clinical applications (6,7).
However, it is found out that bacteria producing L-asparaginase might be affected by various factors in environments (8). Heavy metals are known as effective factors on the metabolism and growth of bacteria (9,10). Herein, L-asparaginase activity in presence of some metal ions, was studied as it has never been reported in in-vivo studies (11). This research focuses on various metal salts implemented for the production of L-asparaginase in broth media. Thus, the work aims to give an overview of the microbial production of L-asparaginase at the presence of some heavy metals.
Soil samples were collected from several different places at a surface of farmlands around the Gharchak regions in November, Tehran, Iran. The samples were collected in sterile plastic bags and carried to the laboratory for microbial isolation. The preliminary screening was performed on nutrient agar plates. Then, the bacterial isolate showing asparaginase activity was detected on M9 modified agar plates supplemented with 0.500 mL of 1% phenol red as pH indicator that changed its color when the isolates catalyzed L-asparagine to L aspartic acid and ammonia (12,13).
The identification of the bacterial isolates was first identified through biochemical experiments and morphological characteristics based on macroscopic and microscopic observation. All the experiments were performed according to Bergey’s manual of determinative bacteriology (14). Phylogenetic analysis was done using 16S rDNA sequencing based on comparing with closest spices deposited in NCBI through Blast alignment software.
Optimization of Enzyme Production
To study enzyme activity in selected bacterium, a synthetic broth medium was designed. M9 medium agar contain asparagus as a nitrogen source, glucose as carbon and K2HPO4 as phosphate sources. Some microelements were also added to the medium to be found vital for growth and enzyme production by bacteria. Initial culture conditions were as follows: pH=7, temperature 30°C, shaking speed 100 rpm and time incubation 48 h. Optimization of culture conditions was performed by the different physical and nutritional factors, such as initial pH (5–10), temperature (20–40°C), agitation speed (80, 90, 100, 110, 120, 130 rpm), carbon sources (glucose, sucrose, fructose, starch, and lactose) and nitrogen sources (yeast extract, beef extract, malt extract, peptone, L-asparagine, L-glutamine and tryptone). All experiments were performed in Erlenmeyer 250 mL containing 100 mL of broth medium as triple replications.
Enzyme Activity Assay
L-Asparaginase enzyme assay was done through the calorimetric method in terms of liberated ammonia from asparaginase activity using Nessler’s reagent (15). After 48 h of incubation, the bacterial cells were removed by centrifuging the culture broth at 7,800 ×g for 15 min and the cell-free extract (0.2 mL) was mixed with 0.8 mL of 0.05 Molar Tris-HCl buffer. Subsequently, 1 mL of 0.04 Molar L-asparagine was added to the mixture and the reaction mixture was incubated for 15 min at 37°C in a water bath shaker. Finally, the reaction was stopped using 0.5 mL of 15% (w/v) trichloroacetic acid (TCA) and the produced ammonia from the enzymatic reaction was measured spectrometrically using Nessler’s reagent at an absorbance wavelength of 500 nm.
The enzyme activity was extrapolated from the liberated ammonia based on a standard curve of ammonium sulfate. The one I.U. (International Unit) of L-asparaginase is equal to one μmol of ammonia released from L-asparagine per minute.
Enzyme activity treated by heavy metal ions was studied. The influence of metal ions such as K+, Na+, Zn2+, Cu2+, Ca2+, Mg2+, Co2+, and Fe2+ was studied in different concentrations (0-3%). After 48 h of incubation at 37°C, inhibitory or stimulatory of the metals were determined for enzyme activity and growth rate of the bacterium.
Isolation and Identification of the Bacterium
Among 75 isolates, only 4 bacterial colonies showed potential L-asparaginase activity. The one that had the most halo red zones on M9 agar was selected for further studies. Figure 1 showes the bacteria with asparaginase activity that changed the pH of the medium when asparaginase released ammonia and diffused vicinity of the colonies.
Identification of Strain MGM1
The phylogenetic analysis demonstrated 97% similarity to 31 Staphylococcus sp, 97% similarity to 33 species of Staphylococcus warneri and 97% similarity to 18 species of Staphylococcus pasteuri. Based on the physicochemical properties and the 16S rDNA sequence, this bacterium belongs to the Staphylococcus genus and designated as Staphylococcus sp. MGM1. The phylogenetic tree (Figure 2) placed the MGM1 strain clearly in distinct branches among the Staphylococcus genus.
Optimization of Enzyme Production
The results of the optimization experiment indicated the desirable physical conditions of each factor as pH=8, temperature 37°C, shaking 100 rpm. To optimize the bacterial growth in synthetic medium, nutritional factors were glucose (as a carbon source), beef extract (as nitrogen source) and K2H PO4 (as phosphate source).
Effect of Heavy Metals on Asparaginase Activity
The results of asparaginase activity at the presence of 8 metal salts showed that Na+, Fe2+, and K+ had a positive effect at 0.5, 0.5 and 1%, respectively while Zn2+ and Mg2+ showed no effect on asparaginase activity. Herein, Cu2+, Ca2+, and Mg2+ had a negative effect up to 1% of their concentration. Co2+ with more than 0.5% w/v was also shown an inhibitory effect.
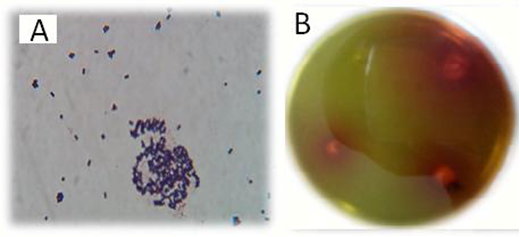
Figure 1. The isolated bacterium graphs. A) gram staining photo and B) asparaginase producing colonies on an M9 agar plate that the color of agar has changed due to the production of ammonia and created a red color around their colonies.
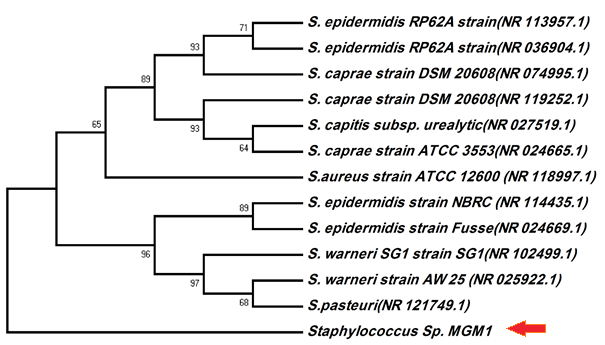
Figure 2. The phylogenetic relationship of Staphylococcus sp.




Figure 3. The effect of metal salts on asparaginase activity in the medium after 48 h incubation at 37°C with agitation speed 100 rpm.
Homology greater than 99% and conformity of the studied bacteria with the suggested bacteria indicates species identification. Homologs of 97% to less than 99% are identified by genus. Homology of 93% to less than 97% indicates a new species or genus, and homology below 93% usually represents a new genus and requires further information to confirm it. Our study reported that MGM1 isolate produced an asparaginase enzyme that is sensitive to metal concentration in the medium. Of course, it must be optimized with salt composition during the fermentation process.
The influence of metals on enzyme activity has already been in line with many authors' ideas. For example, Mase et al., (1995) reported that lipase activity is not affected by Ca2+, Mg2+, Mn2+, Na+, K+, and Cu2+ in Penicillium roqueforti (16). A. Jayaprakash and P. Ebenezer (2012) reported that Calcium chloride appeared to be the best inducer of lipase yielding as much as 100% relative activity followed by mercuric chloride, magnesium chloride and barium chloride (17).
The results showed that Co and Cu repressed enzyme production, which is in accordance with the results of Shora and Ali (2013) showing that Na+, Zn2+, and Cu2+ act as inhibitory for L-asparaginase (18). In contrast, Basha et al. showed stimulatory effect for Mg2+ and inhibitory for Zn2+ on asparaginase isolated from actinomycetes, while our enzyme showed little effect for Mg2+ and no effect for Zn2+ (19). Different ions have different effects on enzymatic activity. This study aimed to investigate the effects of different ions on the activity of the enzyme in the blood and fluid of the body, which affects enzymatic activity. Such compounds are vital elements in the blood and therefore, their effect on the enzyme is very important.
According to the homology and physiological percentages of the bacterium in this study, it is probably a new species of Staphylococcus genus or a strain of Staphylococcus warneri or Staphylococcus pasteuri. And recorded as Staphylococcus sp. MGM1 under the accession number KT361190. The research showed the enzymatic activity of L-asparaginase like other enzymes could be influenced by the various factors. This matter is a critical issue for enzyme reactions, especially, in the case of this study, that the enzyme was admitted as an intravenous drug and contacts with blood components such as cells, ions, proteins, etc. Therefore, it is necessary that enzyme activity be evaluated while being treated by various factors.
The authors thank everyone who helped them with this research.
Authors declared no conflict of interests.
Received: 2019/12/4 | Accepted: 2019/12/30 | ePublished: 2020/01/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






