BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-2211-en.html

 , Jawhar Gharbi
, Jawhar Gharbi 

 2, Mohammed Almalki1
2, Mohammed Almalki1 
 , Khaled Alahmed3
, Khaled Alahmed3 
 , Abdulatheem Ali Brahim4
, Abdulatheem Ali Brahim4 
 , Fatimah Alshakes4
, Fatimah Alshakes4 
 , Manel Ben M'hadheb5
, Manel Ben M'hadheb5 


2- Department of Biological Sciences, College of Science, King Faisal University, Al-Ahsa, Saudi Arabia , jagharbi@kfu.edu.sa
3- Department of Polyclinic, King Faisal University, Al-Ahsa, Saudi Arabia
4- Department of Pediatric, Almoossa Hospital, Al-Ahsa, Saudi Arabia
5- Virology and Antiviral Strategies Research Unit UR17ES30, Higher Institute of Biotechnology, University of Monastir, Monastir, Tunisia
Acute gastroenteritis is one of the most common diseases in humans worldwide, and it has a significant impact on the health of people, especially children. More than 20 agents have been recognized as important causes of this disease. However, most cases of acute viral gastroenteritis are caused by rotavirus, norovirus, adenovirus, and astrovirus. The primary symptoms are vomiting and diarrhea; thus, severe cases can lead to dehydration, and in some cases, it is fatal (1-5).
Deaths from diarrheal diseases in children have essentially declined; however, the fact that the majority of deaths occur in developing countries, especially in Asia, has not changed. It was further estimated that children under 5 years have several episodes of diarrheal diseases each year in underdeveloped countries, in contrast to developed countries where people of all ages have approximately one episode of gastroenteritis each year, and the percentage of hospitalization is high, but only a few patients die. Due to the lack of identification tools for viral detection, it is easy to identify AGEs as clinical entities. Vomiting and diarrhea are the most common symptoms. Still, acute gastroenteritis can be caused by various Enteropathogens, such as viruses, bacteria, and parasites, or a noncontagious cause (6-8).
Gastroenteric viruses have caused around 91% of annual deaths among children aged ≤ 5 years, and the majority of them have occurred in developing countries, especially in Asia (9-11). RoVA, NoV, HAstV, EAdV, and HEV are the most common causes of these infections (12-15). Infections occurred throughout the year but significantly increased by 60% in the winter (16, 17). The main transmission route of enteric viruses causing AGE is fecal-oral (10, 12, 18, 19). It is hard for physicians to identify viral AGE based on only apparent clinical symptoms during the initial diagnosis of the patient's conditions due to the great similarity between viral gastroenteric and bacterial gastroenteritis in symptoms (20, 21).
Gastrointestinal viruses were identified by electron microscopy in the early 1970s, and since then, much has been learned about their essential role in causing epidemic and sporadic gastroenteritis. The rate of detection of gastroenteric viruses such as astrovirus increased by 2-13 % using electron microscopy (20-22). Even though it is limited to reference laboratories, it is still the mainstay of diagnosis (23, 24).
The advent and widespread application of molecular diagnostic techniques, including Multiplex Gastrointestinal Pathogen Panel (GPP) Tests, have led to a vastly improved understanding of the epidemiology, natural history, and clinical manifestations of norovirus infection. Also, characterization of the viral genome has improved diagnostic capability and helps to increase epidemiological surveillance. Moreover, introducing more sensitive techniques, such as the immunoassay technique for antigen detection of pathogenic viruses, has improved the diagnosis of newly recognized viruses (25). In addition, molecular techniques such as reverse transcription-PCR (RT-PCR), endpoint PCR, and real-time PCR have become the gold standard and advanced tool in clinical virology (18). They provide high sensitivity and specificity for identifying any pathogen (6, 7, 15, 26, 27). Finally, based on improved laboratory techniques, the current study aimed to study the epidemiology and the prevalence of the most common gastroenteric viruses, including RoVA, NoVGI, NoVGII, HAstV, EAdV, and HEV among children admitted to Al-Ahsa region - Saudi Arabia, by using multiplex-PCR.
2.1. Samples Collection and Ethical Approval
From December 2021 to June 2022, 92 stool samples were collected from pediatric patients aged ≤7 years who suffering from acute gastroenteritis symptoms. The samples were collected from outpatients and inpatients in the pediatric clinic of King Faisal University (KFU) polyclinic and Almoosa Specialty Hospital (ASH) in the Al-Ahsa region - of Saudi Arabia, respectively. All samples were collected under the supervision of pediatricians, who supervised filing sheets about the parent consent form and the patient clinical information.
Samples were collected in sterile appropriate tubes and conserved before testing at -20°C in the research laboratory. The culture of prototype virus strains as positive controls and sterile DEPC water as negative control were used in the molecular multiplex PCR detection steps. As exclusion criteria, Samples of patients with bacterial and parasite infections were removed from this study.
2.2. Extraction of viral genome (DNA/ RNA) from stool samples:
The protocols for isolation of viral RNA from stool samples and RNA/DNA target detection were adapted from previously published work (12-15). The whole stool was diluted 1:5 (weight per volume) in sterile PBS, homogenized, and clarified by centrifugation at 4000 rpm for 20 min at 4°C. According to the manufacturer's instructions, 100 μL of clarified supernatant was used for RNA extraction using TriZol reagent (Sigma, USA). Thereafter, total DNA was extracted using the DNAbler Extraction Kit (Haven Scientific, KSA). The purity of extracted RNAs and DNAs was controlled by determining OD at 260/280 nm using a spectrophotometer (Thermo Fisher, USA).
2.3. cDNA Synthesis by Reverse Transcription (RT) PCR
The reverse transcription reaction was conducted using the GoScript™ Reverse Transcription kit (Promega, USA). cDNA was synthesized by adding 10 µL of extracted RNA to 10 µL of GoScript™ Reverse Transcription Mixture, which contains 0.5 µL of GoScript Enzyme mix (M-MLV reverse transcriptase and recombinant RNasin ribonuclease inhibitor), 2 µL of Oligo (dT) primer, 2 µL of dNTPs, 2 µL of buffer (MgCl2 + DTT), and 3.5 µL of DEPC water (Promega, USA) (17, 23, 27). RT mixture was placed in thermocycler GeneAmp PCR systems 9700 (Applied Biosystem, USA) and was run with the following of the thermocycler program: primer annealing 37°C for 5 min, extension 42°C for 60 min and inactivation enzyme 70°C for 15 min.
2.4. Multiplex PCR Reaction
Detection of gastroenteric virus genomes was applied using the PCR Master Mix kit (Promega, USA) according to the manufacturer's instructions. PCR was conducted by mixing 10 µL of cDNA and 40 µL of Go Taq PCR Mix, which contains 0.2 µL of Go Taq polymerase, 4 µL of mix primers (Table 1), 2 µL of dNTPs, 4 µL of buffer, and 29.8 µL of DEPC water (17, 23, 27). Multiplex-PCR mixture was placed in GeneAmp PCR thermocycler system 9700 (Applied Biosystems, USA) and was run with the following program: initial denaturation 94°C for 3 min (1 cycle), denaturation 94°C for 30 seconds (35 cycles), primers annealing 45°C for 30 seconds (35 cycles), extension 72°C for 1 min (35 cycles), and final extension 72°C for 7 min (1 cycle). The PCR products were analyzed by 2% agarose gel electrophoresis (ThermoFisher, USA) after staining with Ethidium Bromide (Sigma, USA). Ultraviolet visualized DNA bands of each sample. Band sizes were determined using a 100 bp DNA ladder as a molecular weight standard.
Table 1. Primer sequences used in multiplex-PCR
| Virus | Reference and Target gene** | Polarity (F/R)* | Sequence (5′ to 3′) | size |
| EAdV | Hexon (17) | HexA (F) | gCC gCA gTg gTC TTA CAT gCA CAT C | 300 pb |
| HexB (R) | CAg CAC gCC gCg gAT gTC AAA gT | |||
| NoV GI | VP1 (23) | QNIF4 (F) | CgC Tgg ATg CgN TTC CAT | 510 pb |
| NV1LCR(R) | CTT AgA CgC CAT CAT CAT TTA C | |||
| NoV GII | ORF1-2 junction (23) | QNIF2d (F) | ATg TTC AgR Tgg ATg AgR TTC TCW gA | 829 pb |
| COG2R (R) | TCg ACg CCA TCT TCA TTC ACA | |||
| RoVA | VP7 (1) | Beg 9b (F) | ggC TTT AAA AgA gAg AAT TTC CgT CTg g | 1067 pb |
| End 9b (R) | ggT CAC ATC ATA CAA TTC TAA TCT AAg | |||
| RoVA | VP4 (1) | Con-3 (F) | Tgg CTT CgC CAT TTT ATA gAC A | 676 pb |
| Con-2 (R) | ATT TCg gAC CAT TTA TAA CC | |||
| HAstV | ORF2 (27) | Mon269 (F) | CAA CTC Agg AAA CAg ggT gT | 449 pb |
| Mon270 (R) | TCA gAT gCA TTg TCA TTg gT | |||
| HEV | 5'NCR (30) | HEV (F) | AAg CAC TTC TgT TTC CCC gg | 156 pb |
| HEV (R) | ATT gTC ACC ATA AgC AgC CA |
* F: Forward; R: Reverse, ** VP: Viral Protein, ORF: Open Reading Frame, NTR: Non-Coding Region.
2.5. Statistical Analysis
Student's t-test was used to establish statistical significance results (P < 0.05) using GraphPad Prism 6 Version 6.02.
I During the period between December 2021 and June 2022, all stool samples (n = 92) from pediatric patients who suffered from AGE symptoms were tested using the described molecular method in the Material and Methods section. Pediatric patients infected with AGE were aged ≤7 years, of which 54.3% (50/92) were males and 45.7% (42/92) were females. All pediatric patients were residents of the Al-Ahsa region. All patient clinical symptoms were recorded from the children’s parents and by the pediatrician examinations.
3.1. Etiology of Gastroenteric Viruses among Study Population
Of the total samples, 68.4% (63/92) were positive for gastroenteric virus infections, and 31.5% (29/92) were negative. All target species, except NoVGII, were detected. RoVA was the most detected strain at 54%, followed by HAstV at 27%, EAdV at 22.2%, NoVGI at 9.5%, and HEV at 6.3% (Table 2). The percentage of mono-infection with gastroenteric viruses was much higher than that of co-infection at 79.3% (50/63) and 20.6% (13/63), respectively (Figures 1 and 2).
Table 2. Percentage of mono- and co-infection with gastroenteric viruses among the study population
| Infection Viruses |
Mono-infection, n (%) | Co-infection, n (%) | Total, n (%) |
| RoVA | 36.5% (n=23) | 17.4% (n=11) | 54% (n=34) |
| NoVGI | 9.5% (n=6) | 0 | 9.5% (n=6) |
| EAdV | 11.1% (n=7) | 11.1% (n=7) | 22.2% (n=14) |
| HAstV | 22.2% (n=14) | 4.7% (n=3) | 27% (n=17) |
| HEV | 0 | 6.3% (n=4) | 6.3% (n=4) |
Figure 1. Incidence of mono and co-infection by gastroenteric viruses among children population

Figure 2. Multiplex PCR results of some examples of samples. MW: Molecular Weight of 100 bp DNA Ladder. NC: Negative control. Samples 1 and 2 are positive for HAstV (449 bp), samples 3 and 4 are positive for EAdV (300 bp), sample 5 is negative, and sample 6 is positive for NoVGI (510 bp).
3.2. Prevalence of Gastroenteric Viruses among Children
The highest percentage of positive pediatric patients were in the age group (1Y-2Y) at 41.3% (26/63), followed by (3Y-4Y) at 32% (20/63), (5Y-7Y) at 30.2% (19/63), (6M-12M) at 11.1% (7/63), and (0M-6M) at 5% (Figure 3). All target species, except NoVGII, were detected in the age group (1Y-2Y). Positive males were slightly higher than females at 38% (35/92) and 30.4% (28/92), respectively (Figure 4).
Figure 3. Prevalence of gastroenteric virus infections among children population based on age.
Figure 4. Prevalence of gastroenteric virus infections among children population based on gender.
3.3. Seasonal Pattern of Gastroenteric Viruses among Children
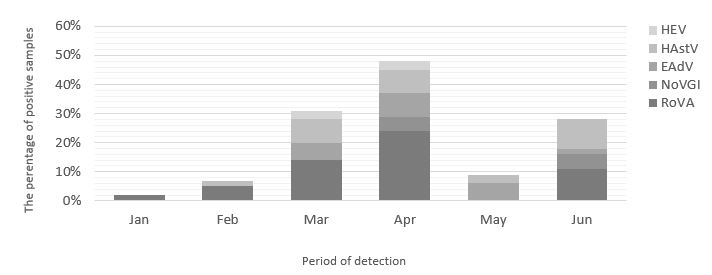
The most outbreaks of gastroenteric viruses were in April at 47.6% (30/63), followed by March at 31.8% (20/63), June at 27% (17/63), May at 9.5%, February at 6.3% (4/63), and January at 1.6% (1/63). In April, all gastroenteric viruses, except NoVGII, were detected, while just one infection with RoVA was detected in January (Figure 5).
Figure 5. Distribution of gastroenteric viruses during the study period.
3.4. Clinical Symptoms associated with Gastroenteric Virus Infections
At the time of hospital or polyclinic admission, all positive pediatric patients with AGE were complaining of different clinical symptoms; 100% (63/63) of pediatric patients had diarrhea, 95.2 % (60/63) had vomiting, 92.1% (58/63) had nausea, and 81% (51/63) had fever. Dehydration and gravity sign appeared in positive pediatric patients at the same percentage at 57.1% (36/63), equally (Figure 6).
Figure 6. Frequency of clinical symptoms associated with the gastroenteric virus infections.
The present study is the first to provide comprehensive data about the etiology and epidemiology of acute viral gastroenteritis among children living in Al-Ahsa province in Saudi Arabia between December 2021 and June 2022. Our results are at variance with previous studies, which showed decreasing of viral gastroenteritis outbreaks among children living in Najran, Saudi Arabia at a rate of 22.1% (72/326) between 2011 and 2012 (28).
By qualitative one-step immune-chromatographic kit, Al Ayed et al. detected in stool samples 56 (17%), 12 (4%), and 4 (1%) for RoV, EAdV, and HAstV, respectively (28). Another previous study in Japan also showed a decrease in AGE widespread among infants in daycare centers (DCCs) from June 1999 to July 2000 (29). Akihara et al. demonstrated that the minority of infants, 30% (276/921), were positive for gastroenteric viruses (29). EAdV was the most prevalent, followed by HAstV, NoV GII, and SapV, represented 39% (n = 107), 24% (n = 67), 21% (n = 59), and 9% (n = 24), respectively (29). In the present study, viral gastroenteritis was highly prevalent among children living in Al-Ahsa province, Saudi Arabia, where the majority of pediatric patients were positive for viral AGE at 68.4%. All RoVA, HAstV, EAdV, NoVGI, and HEV were detected at 54%, 27%, 22.2%, 9.5%, and 6.3%, respectively.
Previous studies showed close percentages of co-infections and mono-infections of gastroenteric viruses among pediatric inpatients in a Hospital‑based Study in India (30). They detected RoV, EAdV, NoV, HAstV, and HuSaV at rates of 21% (17/80) and 34% (27/80), respectively (30). The highest co‑infection was observed with EAdV + NoV at 20%, followed by EAdV +RoVat at 14%, HuSaV + RoV at 5%, and NoV + EAdV + RoV at 5%. Real-Time PCR was used for gastroenteric virus detection in stool samples (30). The present study observed that mono-infection with gastroenteric viruses was much higher than co-infection at 79.3% and 20.6%, respectively. The mono/ co-infection of RoVA was the most detected strain at 36.5% (n=23) and 17.4% (n=11), respectively.
During the period 2017 and 2018, previous studies in hospitalized Finnish Finland observed that pediatric patients aged between (2Y and 5Y), were more susceptible to infection with RoV, NoV, and HuSaV at a rate of 66% (115/175) than children aged between (6Y and 16 Y) (31). Females made up 52% of viral AGE cases (31). During the period Jun 2006 and Dec 2007 in the United Kingdom (UK), previous studies showed that positive cases with community-acquired AGE (CA-AGE) and healthcare-associated AGE were aged between (10 M and 16Y) at a rate of 59% (339/576) (31, 32). In the present study, the highest percentage of positive children with viral AGE who living in Al-Ahsa province, Saudi Arabia, was forage group (1Y-2Y) at 41.3%, while the lowest percentage was for the age group (0M-6M) at 5%. Males were more infected than females at 38% and 30.4%, respectively.
Previously published studies showed the prevalence of infections of RoVA among children living in Riyadh, Saudi Arabia, occurred throughout the year with no clear significant seasonal peaks during the period 2005 and 2010 (33). That study used ELISA and molecular methods to detect and characterize RoVA strains in stool samples (33-35). In the present study, April was the most outbreaks of viral AGE among Al-Ahsa children at a rate of 47.6% (30/63), followed by March at 31.8% (20/63). February and January had the lowest rate of outbreaks at 6.3% and 1.6%, respectively.
A previous study in Najran, Saudi Arabia, showed that all positive children with viral AGE 100% (n = 72) had semi-liquid and watery diarrhea, and 80% had vomiting during the period between 2011 and 2012 (28). In addition, previous studies in the UK showed that all positive children with viral AGE at 59% (339/576) were accompanied by diarrhea, fever, vomiting, and dehydration, equally from 2006 to 2007 (32). A high percentage of viral AGE cases at 81% (274) were located in acute general medical and surgical wards, while 20% (n = 69) were located in critical care units (32).
In the present study, at the time of hospital/ polyclinic admission, all pediatric patients complained of different clinical symptoms of viral AGE. All positive children with viral AGE at 100% had watery diarrhea, and 95.2 % had vomiting. While 92.1% of positive children with viral AGE had nausea, and 81% had a fever. Dehydration and gravity signs appeared in positive children at the same percentage at 57.1%, equally. Children who had dehydration needed to be hospitalized because all of them were in the acute phase of the disease and stayed in pediatric medical word (PMW) to take IV fluid and IV antihistamine. The present work represents the first study of the epidemiology of AGE in children in the province of Al-Ahsa. Still, the number of samples and expansion of the study over a long period is recommended in future studies.
This study showed that gastroenteric virus infections, associated explicitly with RoVA, are highly prevalent among children aged in-group (1Y-2Y) living in Al-Ahsa province, Saudi Arabia. These results could reduce data limitations about viral etiology and incidence of children's acute gastroenteritis in Al-Ahsa province, Saudi Arabia, but more investigations are needed.
We thank all the parents of the pediatric patients who participated in this study. In addition, all thanks and appreciation to all the medical staff in the pediatric department of Al-Moossa Hospital and pediatricians in the polyclinics of KFU for their assistance in collecting samples.
Ethics approval
The ethical approvals were obtained from the ethics committee of the Deanship of Scientific Research of KFU for samples collected from the KFU polyclinic patients (Agreement #2020-9415) and from Almoosa Academic Affairs for samples collected from Almoosa Hospital patients (IRB #2022-ARC-22.02.05).
Conflicts of Interest
The authors declare no conflict of interest.
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [GRANT Nb. 4269].
Received: 2023/08/13 | Accepted: 2023/11/15 | ePublished: 2023/11/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |