BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1572-en.html
2- Department of Biology, Rasht Branch, Islamic Azad University, Rasht, Iran , asadpour@iaurasht.ac.ir
Keeping pets is associated with physical and mental benefits due to positive impacts on the quality of life of people. However, this association between humans and animals may lead to the transmission of various pathogens to the owners and pose risks to public health (1). After dogs and cats, ornamental birds are the world's third most common type of pet (2). Ornamental birds can harbor some pathogens and transmit them to their owners. Although the ownership of these birds is not without danger, many people take care of ornamental birds in their homes and thus expose themselves to various common bacterial, protozoan, fungal, viral, or parasitic diseases (3). Escherichia coli is common in the gastrointestinal tract of humans and animals. Still, some strains of this bacterium, known as avian pathogenic E. coli (APEC), can cause intestinal and extraintestinal diseases in humans and mammalian and avian species and lead to serious economic losses and public health problems (1). APEC isolates are genetically similar to human extraintestinal pathogenic E. coli (ExPEC), which includes uropathogenic E. coli strains. They may have a wide range of virulence factors and can be transmitted to humans, particularly through the food chain or through direct contact with birds, and cause extraintestinal infections (4-6). Some human extraintestinal pathogenic E. coli strains have iss gene in their genomes, which is located on plasmid ColV, a huge virulence plasmid typical of avian pathogenic E. coli strains. This indicates that the exchange of plasmids and, consequently, the exchange of those virulence genes, are possible between APEC and UPEC strains (6).
Therefore, APEC strains can be a repository for virulence genes that are pathogenic to humans. The distribution of virulence genes varies in APEC, and fecal E. coli (AFEC) isolates. Studies have shown that pathogenic strains are different from fecal strains due to having a set of specific genes located on chromosomal and plasmid pathogenic islands (7-9).
The results of a study showed that the acquisition of APEC plasmids by AFEC increases its ability to kill chicken embryos, grow in human urine, and colonize rat kidneys (10). In a study by Johnson et al. (2008), out of 46 genes studied, 5 genes (iutA, iss, ompT, iroN and hlyF) were the most associated genes with pathogenesis, and a pentaplex panel was suggested as the minimum criterion for differentiating between APEC strains (8). Among these genes, iss is an important gene in APEC, which encodes the Iss protein, increases serum survival, and prevents the bactericidal activity of complement systems. The ompT gene encodes an outer membrane protease (OmpT) that activates plasminogen to plasmin and plays an important role in the degradation of proteins. The hlyF gene is expressed during extraintestinal infection and leads to the formation of outer membrane vesicles (OMV), which are involved in the transmission of bacterial virulence factors and promote the pathological process in the infected host. The iroN and iutA virulence genes encode outer membrane receptors for iron, siderophore, and aerobactin, respectively (11, 12).
In addition to the zoonotic importance of these bacteria, the spread of different antibiotic-resistant bacterial strains has become a major concern for the World Health Organization. Bacterial resistance, which is increasing through the production of broad-spectrum beta-lactamases, has recently been suggested as a global therapeutic problem (13-15). Numerous studies have been performed regarding E. coli antibiotic resistance status, indicating rapid transfer of antibiotic resistance genes between strains (especially the plasmid-encoding ones) (16, 17). Antibiotic resistance in E. coli has reached a point that is identified as a serious clinical challenge in humans. Studies have revealed that domestic and wild animals in contact with humans show similar antibiotic resistance (16). Studies on antibiotic-resistant E. coli isolates obtained from birds in different parts of the world have shown diverse results regarding the prevalence of resistant E. coli in different regions (18).
Considering that ornamental birds can act as a reservoir for the development of acute and antibiotic-resistant E. coli, obtaining information about the status of virulence potential and drug resistance in ornamental birds is essential. Therefore, the present study was conducted to investigate the pattern of antibiotic resistance and virulence of E. coli isolates obtained from ornamental birds in Guilan province, northern Iran.
Isolation of bacteria
In order to isolate E. coli, cloaca swabs of 80 apparently healthy ornamental birds, including cockatiel (45 isolates), budgerigar (19 isolates), and finch (16 isolates), were collected in Rasht in 2021. For this purpose, the area around the cloaca was first disinfected with 70% alcohol. In order to isolate E. coli, the target swab was cultured on MacConkey, and EMB agar and pure colonies were identified using biochemical tests (1).
Antibacterial Resistance of Test Bacteria
Antibiotic resistance of isolates was determined by the disk diffusion method using antibiotic disks. For this purpose, 100 μL of microbial culture with turbidity equivalent to 0.5 McFarland concentration was spread on a Müller-Hinton agar (Quelab) medium, and then antibiotic disks (antibody of medicine) were placed on the culture. The plates were then incubated at 37°C for 24 hours. The diameters of the missing halos were reported as sensitive (S), resistant (R), and intermediate sensitivity (I) according to the 2020 CLSI standard table.
The studied antibiotics included amikacin (30 µg), gentamicin (10 µg), imipenem (10 µg), cefotaxime (30 µg), ampicillin (10 µg), amoxiclav (20/10 µg), aztreonam (30 µg), chloramphenicol (30 µg), trimethoprim-sulfamethoxazole (1.25/23.75 µg), tetracycline (30 µg), penicillin (10 µg), ampicillin-sulbactam (20 µg), piperacillin-tazobactam (110 µg) and cefepime (30 µg).
To investigate the phenotypic ability to produce broad-spectrum beta-lactamase, the combined disk method was utilized using cefotaxime and cefotaxime-clavulanic acid disks. If the non-growth halo around the disks containing clavulanic acid was greater than or equal to five millimeters in diameter of the zone around antibiotics without clavulanic acid, the desired strain was considered as ESBL producer according to CLSI standard (19).
DNA Extraction and Virulence Genotyping
The boiling method was used to extract DNA. To apply this method, a colony was removed from the nutrient agar medium, dissolved in 1.5 mL BHI broth, and incubated for 20 hours. Then, the microtubes were centrifuged for 5 minutes at 5000 rpm. The supernatant was discarded, and the pellet was suspended in 500 μL of distilled water. The resulting suspension was boiled for 5 minutes for cell lysis and then centrifuged. The supernatant contained the extracted DNA.
To evaluate the virulence of isolates, the frequency of five E. coli virulence genes, including iroN, ompT, hlyF, iss, and iutA in fecal isolates of ornamental birds, were examined by PCR according to the study of Johnson et al. (2008) (8). The list and oligonucleotide sequence of primers used in this study are presented in Table 1.
The polymerase chain reaction was performed in a volume of 25 μL, each reaction containing 12.5 μL of Master Mix (CinnaGen) (containing 10X PCR Buffer, MgCL2, dNTP at a concentration of 20 mmol and 1 unit of Taq polymerase enzyme), 1 μL of each of the primers at a concentration of 10 picomoles, 2 μL of template DNA and 8 μL of sterile distilled water. The polymerase chain reaction for each gene was performed at 95°C for 5 minutes, 95°C for 1 minute, 63°C for 40 seconds, 72°C for 50 seconds and 72°C for 7 minutes. Electrophoresis of PCR products was performed on 1% agarose gel.
Statistical analysis
The correlation between antibiotic resistance and the frequency of virulence-associated genes in test bacteria was analyzed using SPSS software and the Chi-square test. P£0.05 was considered significant.
Table 1. List and oligonucleotide sequence of primers used in this study
| Gene | Function | Primer Sequence | Product Length (bp) |
| iroN | Salmochelin siderophore receptor gene | F: 5′- AATCCGGCAAAGAGACGAACCGCCT -3′ R: 5′- TTCGGGCAACCCCTGCTTTGACTTT-3′ |
553 |
| Hly-f | Putative avian hemolysin | F: 5′- GGCCACAGTCGTTTAGGGTGCTTACC -3′ R: 5′- GGCGGTTTAGGCATTCCGATACTCAG -3 |
450 |
| iss | Episomal increased serum survival gene | F: 5′ CAGCAACCCGAACCACTTGATG- 3' R: 5′- AGCATTGCCAGAGCGGCAGAA -3' |
323 |
| Ompt | Episomal outer membrane protease gene | F: 5′- TCATCCCGGAAGCCTCCCTCACTACTAT -3' R: 5′- TCATCCCGGAAGCCTCCCTCACTACTAT -3' |
496 |
| Iut-A | Aerobactin siderophore receptor gene | F: 5′- GGCTGGACATCATGGGAACTGG -3' R: 5′- GGCTGGACATCATGGGAACTGG -3' |
302 |
Out of 80 cultured isolates, 32 E. coli isolates were obtained from fresh feces of ornamental birds. The isolates had reddish-pink colonies on MacConkey medium, metallic green shine on EMB medium, gram-negative bacilli, oxidase negative, catalase-negative, Simmons citrate negative, and urease negative. In the TSI medium, hydrogen sulfide production was negative, gas production was positive, and the medium's color was acid-acid (A/A). Positive indole production and bacterial motility in the SIM medium were observed by creating turbidity in the culture path. In MR-VP, the result of the MR test was positive, and the result of the VP test was negative. Of these, 16 isolates were obtained from cockatiel feces, 6 isolates from budgerigar, and 10 isolates from finch.
Antibacterial Susceptibility of Isolates
Antibiogram testing was performed on all E. coli isolates. In this study, 90% of isolates were resistant to at least one antibiotic, while 14 isolates (43.75%) had multiple drug resistance (MDR). The isolates were most sensitive to gentamicin and then to imipenem and cefotaxime. In isolates, the highest level of resistance was resistance to penicillin (90.63%) and piperacillin-tazobactam (68.75%). Moreover, a broad-spectrum beta-lactamase-producing isolate was identified among cefotaxime-resistant isolates. The results of antibacterial resistance in fecal E. coli are shown in Table 2.
Virulence Genotyping
In PCR reaction, the frequencies of iroN, ompT, hlyF, iss, and iutA genes in fecal isolates of ornamental birds were 9 (26.12%), 11 (34.37%), 13 (40.62%), 12 (5), and 14 (43.75%), respectively. Agarose gel electrophoresis of the PCR product of these genes is shown in Figures 1-3.
More than one virulence gene was identified in 19 isolates. Based on the method reported by Johnson et al. (2008), isolates of at least 4 of the 5 studied genes identified in the PCR reaction were considered APEC. Accordingly, out of 32 fecal isolates, 8 isolates (5 isolates carrying 4 virulence genes and 3 isolates carrying 5 virulence genes) were identified as APEC (8).
Table 2. Results of antibacterial resistance in E. coli isolates obtained from feces of ornamental birds
| Resistant, N (%) | Intermediate, N (%) | Sensitive, N (%) | Antibiotic |
| 4 (12.5) | 0 | 28 (87.5) | Gentamicin |
| 2 (6.25) | 4 (12.5) | 26 (81.25) | Imipenem |
| 8 (25) | 0 | 24 (75) | Chloramphenicol |
| 4 (12.5) | 2 (6.25) | 26 (81.25) | Cefotaxime |
| 29 (90.63) | 0 | 3 (9.37) | Penicillin |
| 12 (37.5) | 0 | 20 (62.5) | Aztreonam |
| 6 (18.75) | 6 (18.75) | 20 (62.5) | Ciprofloxacin |
| 7 (21.87) | 2 (6.25) | 23 (71.87) | Levofloxacin |
| 17 (53.12) | 6 (18.75) | 9 (28.13) | Tetracycline |
| 5 (15.62) | 5 (15.62) | 22 (68.75) | Trimethoprim/sulfamethoxazole |
| 3 (9.37) | 4 (12.5) | 25 (78.13) | Colistin |
| 7 (21.87) | 4 (12.5) | 21 (62.63) | Cefepime |
| 12 (37.5) | 7 (21.87) | 13 (40.63) | Streptomycin |
| 22 (68.75) | 0 | 10 (31.25) | Piperacillin-Tazobactam |
| 5 (15.62) | 7 (21.87) | 20 (62.5) | Ampicillin-Sulbactam |
Figure 1. Agarose gel electrophoresis of iroN (Right) and ompT (Left) PCR products with approximate sizes of 533 bp and 496 bp, respectively.
Figure 2. Agarose gel electrophoresis of hlyF (Right) and iss (Left) PCR products with approximate sizes of 450 bp and 323 bp, respectively.
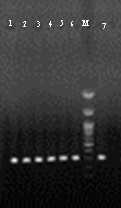
Figure 3. Agarose gel electrophoresis of iutA gene PCR products with an approximate size of 302 bp.
Correlation between antibiotic resistance and frequency of virulence genes
All imipenem, cefotaxime, and colistin-resistant isolates were recognized as APEC. The frequencies of iss, iutA, hlyF, and ompT were significantly higher in MDR isolates (P<0.05), and each penicillin, piperacillin-tazobactam, and tetracycline-resistant isolate harbored at least 2 virulence genes.
Excessive use of antibiotics, in addition to high costs and various side effects, leads to a very serious problem known as antimicrobial resistance. Due to the fact that ornamental birds can potentially play a role in the spread and transmission of antibiotic-resistant E. coli to humans, it is necessary to know the status of antimicrobial resistance in ornamental birds and to prevent the use of antibiotics in the treatment or prevention of disease in these birds. In the present study, 40% of gram-negative bacilli obtained from the feces of ornamental birds were E. coli. This amount is higher than most of the frequencies of this bacterium reported in previous studies. For example, in the study of Tahmasebi et al. (2014), the frequency of fecal E. coli isolates in ornamental birds was 12.1% (18). Some other studied reported this amount as 36.1% (20), 9.1% (21), 35.2% (22), and 10% (1). The closest result to the present study was reported by Sigirci (2) et al. (2020), in which the frequency of E. coli in canary and finch feces was 37.7% (2).
In this study, 90% of isolates were resistant to at least one investigated antibiotic, and 14 isolates (43.75%) showed multiple drug resistance. Test isolates showed high resistance to penicillin (90.63%) and piperacillin (75%), while gentamicin, imipenem, and cefotaxime were the most effective antibiotics.
In studies conducted in different parts of the world, antibiotic-resistance status has been different in E. coli isolates obtained from different birds. In some studies, a very high level of antibiotic resistance (23), and in others, a lower prevalence has been reported (24-27). A report on E. coli isolates obtained from Czechoslovakian pigeons showed a low antibiotic-resistance level of 5.1% (27). On the other hand, a high rate of antibiotic resistance was reported in a study conducted by Askar et al. on domestic pigeons in Turkey. The highest resistance of E. coli isolates was observed against ampicillin-sulbactam (70%), oxytetracycline (64%), and nalidixic acid (49%) (23). This high resistance rate to tetracycline is consistent with the present study. High resistance to tetracycline (22%) has also been reported in a study conducted by Dolejská et al. on E. coli isolates obtained from black-tailed chickens. However, contrary to our study, no resistance was observed concerning third-generation cephalosporins ceftriaxone and ceftazidime (28). In another study by Silva et al., contrary to our study, resistance to third-generation cephalosporins, ceftriaxone, and ceftazidime was not detected in diarrheagenic E. coli in Brazil. Among these isolates, amikacin had the lowest resistance rate at 36.8%. In accordance with this result, the high efficacy of tested aminoglycoside (gentamicin) has been detected in the present study (26). Furthermore, the high resistance to penicillin (ampicillin and amoxicillin) and high efficacy of gentamicin reported by Beleza et al. are in agreement with the present study (21).
Studies in recent years have reported higher antibiotic resistance in E. coli isolates obtained from ornamental birds. In the study by Giacopello et al. (2015), all E. coli isolates obtained from canary feces were resistant to penicillin, amoxicillin, and erythromycin. These isolates showed high sensitivity to ciprofloxacin and enrofloxacin. High resistance to penicillin and high sensitivity of isolates to fluoroquinolones are consistent with the results of the present study (22).
In the study of Sigirci et al. (2020), high levels of resistance to tetracycline (84%) and sulfamethoxazole-trimethoprim (46%) were reported in E. coli isolates obtained from the feces of ornamental birds (2). Pontes et al. (2018) also reported high levels of resistance to amoxicillin (82%), ampicillin (79%), streptomycin (67%), and tetracycline (41%) in E. coli isolates obtained from the Dutch bride. Furthermore, 59% of isolates showed multiple drug resistance in this study (1). In the present study, we detected one ESBL-producing E. coli isolate from companion birds. Transfer of beta-lactamase-producing E. coli between humans and yellow-tailed hens was previously reported in a study in France (29).
In comparison with drug resistance, the virulence of E. coli fecal isolates in ornamental birds has been investigated in fewer studies. In the present study, the frequencies of iroN, ompT, hlyF, iss, and iutA genes in 32 E. coli isolates of ornamental birds were 28.12%, 34.37%, 40.62%, 37.5%, and 43.75%, respectively, 8 isolates (25%) were identified as APEC, and in 19 isolates, more than one virulence gene was identified.
In the study of Pontes et al. (2018), out of 4 E. coli isolates, 2 isolates (50%) were identified as APEC (1). In another study in Iran by Mohammadzadeh et al., out of 117 feces samples from the cloaca of pigeons in Tehran, the frequency of stx2, stx1, and hly genes were reported at 6.18, 3.09, and 2.06%, respectively. The hlyA gene was not present in any isolates (30).
In this study, E. coli isolates obtained from ornamental birds showed antibiotic resistance and virulence potential of fecal E. coli isolates in ornamental birds in Rasht. The release of pathogenic and drug-resistant strains into the environment can endanger the health of owners and the whole society.
This article is the result of the first author's master's thesis. The authors would like to thank the Islamic Azad University, Rasht Branch, for their support.
Parastoo Akbari was involved in collecting samples, conducting experiments, and writing the manuscript. Study design, analyzing the results, and correcting of the manuscript were done by Leila Asadpour.
The authors didn't receive any financial support.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2021/12/2 | Accepted: 2022/05/18 | ePublished: 2022/08/8
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |











