BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1070-en.html
2- Department of Microbiology, Stamford University Bangladesh, 51, Siddeswari Road, Dhaka-1217, Bangladesh ,
3- Centre for Advanced Research in Sciences, University of Dhaka, Dhaka-1000, Bangladesh
.
From the last couple of decades, the inherent therapeutic properties of medicinal plants are a focus in the scientific community because of their potentiality against drug-resistant micro-organisms (1). The increasing use of plant-based chemicals is established by WHO and it is estimated that around 80% of the individuals in the developing country are using plant-based bioactive components for their primary healthcare (2). There is a global concern that around 60,000 plant species would be extinct by the middle of twenty-first century if the present trend persists (3). Therefore, exploration of the therapeutic potential of biologically active components before they become extinct is a must. Plant-based biomolecules have been considered as an alternative to antibiotics due to their effectiveness against antibiotic-resistant microorganisms (4). In the last ternary decades, the pharmaceutical industries produced a large number of new antibiotics but drug resistance has been increasing simultaneously. Consequently, medicinal plants and herbs which are deemed to be candidates in place of conventional antibiotics have gained increasing interest. In addition, the bioactive medicinal compounds in plants are more protected and less toxic than the expensive synthetic drugs which have lots of adverse effects (1, 5).
The Piper betle, usually called as betel, is an evergreen climbing plant with glossy heart-shaped leaves and white catkin and belongs to the Piperaceae family (6). The plants are extensively grown in South and Southeast Asia including Bangladesh, India, Sri-Lanka, Malaysia, Thailand, and Taiwan. There are lots of common names as betel in English, Paan in Bangladesh and India, Phlu in Thailand and Soroh in Bahasa Indonesia (7). A strong pungent aromatic flavor of betel leaves is the reason why people from Bangladesh and from Asian countries use it as a mouth freshener after a meal or appetizer. Usually, 60-70% Bangladeshi consumes betel leaves frequently in their social, cultural, and religious festivals as marriage, puja, shraddha, etc. (8). Betel leaves have different therapeutic activities as antidiabetic, antiulcer, antiplatelet aggregation, antifertility, cardiotonic, antitumor, antimutagenic, respiratory depressant, carminative, stomachic, anthelmintic, tonic, and aphrodisiac (9-13). They are used in traditional treatment for various diseases like bad breath, boils, and abscesses, conjunctivitis, constipation, headache, hysteria, itches, mastitis, mastoiditis, leucorrhoea, ringworm, swelling of gum, rheumatism, abrasion, cuts and injuries, etc (14).
In the present research, an endeavor was made to explain the antibacterial activity of ethanol and acetone extracts of P. betel leaves from two different locations namely Barguna and Moheshkhali against the common foodborne bacteria. We also attempted to profile the components of phytochemicals, and to obtain functional groups of P. betle leave extracts by FT-IR spectral peak values.
Plant
Fresh and healthy P. betle leaves were collected from two different areas (Barguna and Moheshkhali) of Bangladesh. The images of those leaves were shown in Figure 1.
Figure 1. Representative images of betel leaves collected from Barguna area (A) and Moheskhali area (B).
Test Organisms and Maintenance
Our study conducted with three bacterial strains available at the Department of Microbiology, Primeasia University and they were Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 6538) and Bacillus cereus (ATCC 14579). Their stock cultures were preserved in media containing 20% glycerol in cryogenic vials and stored at -81°C. Stock cultures were revived and cultured on Tryptic Soy Agar (TSA) slant and kept at 4°C for study. Freshly grown overnight cultures were used for regular work.
Preparation of Extracts of Betel Leaves
Betel leaves that were collected from Barguna and Moheshkhali were properly washed with distilled water and dried at 45°C for 48 h in an oven. The dried leaves were crushed in a mortar and pestle, and further ground to get fine dry powder. Twenty grams of fine betel leaf powder was soaked in 80 mL of 95% acetone and another twenty grams were soaked in one more 80 mL of 95% ethanol in a sterilized bottle. Both were kept in a reciprocal shaker chamber (WIS-10, wisecube, Germany) at 100 rpm for overnight at room temperature. The acetone and ethanol fractions were separated using sterilized cheesecloth and filtered through sterilized Whatman filter paper (No.01). The collected filtrates were kept in the hot dry oven (ED53, Binder, Germany) at 50°C to reduce the volume of those filtrates. The final concentration of the extracts were prepared as 100 mg/mL in acetone and ethanol, separately and kept at 4°C for successive studies (15).
Phytochemical Screening
The phyto-constituents as alkaloids, flavonoids, saponins, steroids, tannins, terpenoids, glycosides, phenols, proteins and carbohydrates of acetone and ethanol extracts of P. betle leaves were analyzed following a previous study (16).
Detection of Antibacterial Susceptibility using Agar Well Diffusion Method
Both acetone and ethanol extracts of P. betle leaves were tested against selective bacterial strains to determine the antibacterial activity by agar well diffusion method. In brief, pure bacterial isolates were sub-cultured in Tryptic Soy Broth (TSB) at 37°C for 24 h and following incubation, the confluence of bacterial culture was checked and adjusted with 0.5 standardized McFarland solutions. The plates were streaked using a sterile cotton swab soaked with culture evenly in three directions keeping the loop at a 60° angle on the surface of the Muller Hinton Agar (MHA) plate. Before spreading, the surplus suspension was removed from the cotton swab by pressing the swab on the inside of the test tube body. After inoculation, plates were dried and wells of 8 mm diameter were made using a sterile metal borer. Subsequently, 100 µL volumes of 1 mg/mL of each extract were poured in properly labeled respective wells and ciprofloxacin was used as a positive control. Those plates were kept in a laminar airflow for 30 min for diffusion to take place and incubated at 37°C for 24 h. After incubation, the diameter of zone of inhibition (ZOI) was measured in millimeters around the wells.
Minimum Inhibitory Concentration (MIC) using Micro-broth Dilution Assay
The broth microdilution method was performed to measure the MIC of the extracts according to NCCL standard (17) using sterile 96-well plates. In brief, a dilution series in sterile MHB of 100 µL of each extract was freshly prepared in micro-dilution plate and 100 µL of selective bacterial culture was inoculated and gently mixed. The final concentrations of the extracts ranged from 34 mg/mL to 0.065 mg/mL. The plates were incubated at 37°C for 24 h and consequently, the presence and absence of turbidity of growth was observed. The MIC was shown as the lowest concentration at which visible growth was absent. The tested extracts were screened in triplicate against each organism.
Fourier Transform-infrared (FT-IR) Spectroscopic Analysis
FT-IR spectra of the compounds were measured using IR grade potassium bromide (KBr). The compounds were separately mixed with 200 mg KBr to obtain round disc with the help of the hydraulic press. The round disc was later subjected to FT-IR in the range of 4000-400 cm-1 using Perkin Elmer spectrophotometer (Germany) with FT-IR paragon 2 software.
Comparative Evaluation of Phytochemical Properties of Betel Leave Extracts
The phytochemical properties of both acetone and ethanol extract of Barguna and Moheshkhali P. betel leaves were summarized in Table 1. All those extracts showed the presence of steroids, diterpenes, tannin, alkaloids, phenols, phytosterol, coumarin, and leucoanthocyanin. Interestingly, carbohydrate, saponins, flavonoids by Zn dust test were found in Barguna betel leave extracts only. However, phlobatannins, cardial glycosides, and proteins were not found in any of the extract.
Table 1. Properties of phytochemicals in acetone and ethanol extracts of P. betle leaves collected from Barguna and Moheshkhali, Bangladesh.
| Name of Microorganisms | Acetone | Ethanol | ||||
|---|---|---|---|---|---|---|
| Barguna | Moheskhali | Borguna | Moheskhali | |||
| Steroids | +++ | +++ | +++ | +++ | ||
| Diterpenes: Copper acetate test | ++ | +++ | ++ | +++ | ||
| Phlobatannins | - | - | - | - | ||
| Tannin | Lead acetate test | +++ | +++ | +++ | +++ | |
| FeCl3 | ++ | +++ | ++ | +++ | ||
| Cardial Glycosides: Keller-Killani test |
- | - | - | - | ||
| Flavonoid: | Alkaline reagent test | - | - | - | - | |
| NH4OH | ++ | ++ | ++ | ++ | ||
| Zn dust test | + | - | + | - | ||
| Phenols: FeCl3 test | ++ | +++ | ++ | +++ | ||
| Carbohydrate | ++ | - | ++ | - | ||
| Phytosterol: Salkowski’s test | +++ | +++ | +++ | +++ | ||
| Alkaloids: Wagner’s test | ++ | ++ | ++ | ++ | ||
| Coumarin | ++ | ++ | ++ | ++ | ||
| Leucoanthocyanin | ++ | ++ | ++ | +++ | ||
| Saponin: Foam test | + | - | + | - | ||
| Proteins | - | - | - | - | ||
Comparative Evaluation of Antibacterial Activity of Betel Leave Extracts
In this study, the antibacterial activity of the acetone and ethanol extracts of Barguna and Moheshkhali betel leaves was evaluated using agar well diffusion method by the diameter of the (ZOI) (Figure 2). The acetone and ethanol extracts showed various degrees of ZOI against the foodborne pathogens, B. cereus, S. aureus and E. coli. But, betel leaf extracts from Barguna and Moheshkhali showed very similar results against those organisms. With 100 mg/mL concentration, the Moheshkhali leaves showed the highest activity against B. cereus and the ZOIs were 23 mm and 24.7 mm in acetone and ethanol extracts, respectively. However, 23 and 22 mm ZOIs were observed with acetone and ethanol extracts of Barguna leaves, respectively against the same organism. Very interestingly, the commercial antibiotic, ciprofloxacin presented a 16.5 mm ZOI against B. cereus. Besides, ciprofloxacin showed the highest antibacterial activity that is 23 mm ZOI against S. aureus, where, acetone and ethanol extract from Barguna showed 18.7 mm and 20 mm ZOIs, respectively and the extracts from Moheshkhali showed 20.2 mm and 22 mm ZOI, respectively. The clear zones against E. coli were observed as 15.7 mm and 16 mm by acetone and ethanol extracts of Barguna leaves, respectively where, Moheshkhali leaves showed 18 mm and 14.2 mm ZOIs, respectively. In this experiment, acetone and ethanol were used as solvent control against all the tested organisms.
MIC of Betel Leave Extracts
The MIC of acetone and ethanol extracts of betel leaves collected from Barguna and Moheshkhali were tested against three bacterial species presented in Table 2. All the tested extracts showed concentration-dependent growth inhibition of the tested organisms. MIC was observed with 2.12 mg/mL concentration in acetone extract of Barguna betel leaves against any of the three bacterial species. Whereas, MIC values of ethanol extracts of Barguna betel leaves showed 2.12 mg/mL against B. cereus, and 4.25 mg/mL against both S. aureus and E. coli. Acetone extracts of Moheshkhali betel leaves showed MIC at 2.12, 8.5 and 4.25 mg/mL against B. cereus, S. aureus, and E.coli, respectively. Additionally, the MIC of ethanol extracts of Moheshkhali betel leaves was 4.25 mg/mL against both B. cereus and S. aureus, but 2.12 mg/mL against E. coli.
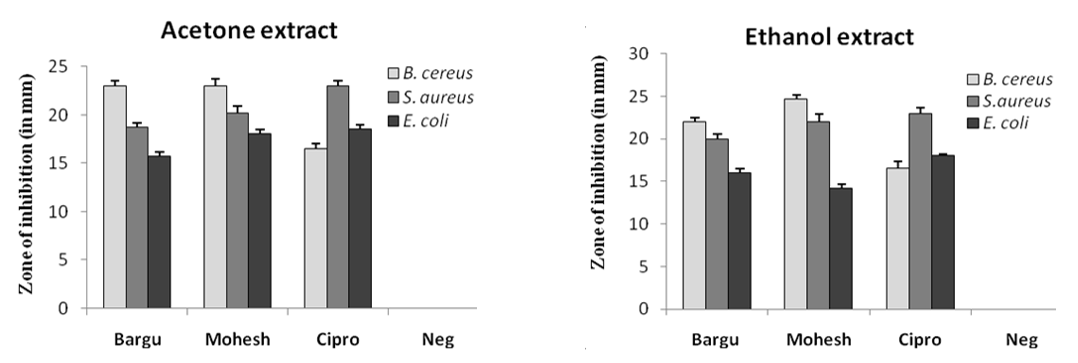
Figure 2. ZOI (in mm) of acetone and ethanol extracts of P. betle leaves against three bacterial species where Barguna, Moheshkhali, Ciprofloxacin, Negative control were denoted as Bargu, Mohesh, Cipro and Neg, respectively.
Table 2. MIC of acetone and ethanol extracts of Barguna and Moheshkhali betel leaves against three selected bacterial pathogens.
| Name of microorganisms | Acetone (MIC) mg/mL | Ethanol (MIC) mg/mL | ||
| Barguna | Moheshkhali | Barguna | Moheshkhali | |
| B. cereus | 2.12 | 2.12 | 2.12 | 4.25 |
| S. aureus | 2.12 | 8.5 | 4.25 | 4.25 |
| E. coli | 2.12 | 4.25 | 4.25 | 2.12 |
Biophysical Characterization of Betel Leave Extracts
The FT-IR spectrum of the active ingredients of Barguna and Moheshkhali betel leave extracts was mostly comparable. The FT-IR spectrum of the extracts was presented in Figure 3. Their peak values corresponding to the functional groups were summarized in Table 3 and 4. However, we did not observe any peak characteristic of triple bonds in the value range of 2500 to 2000.
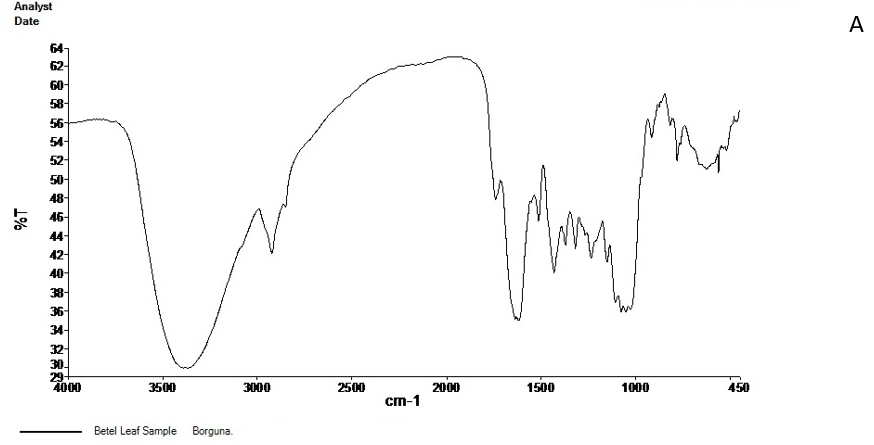
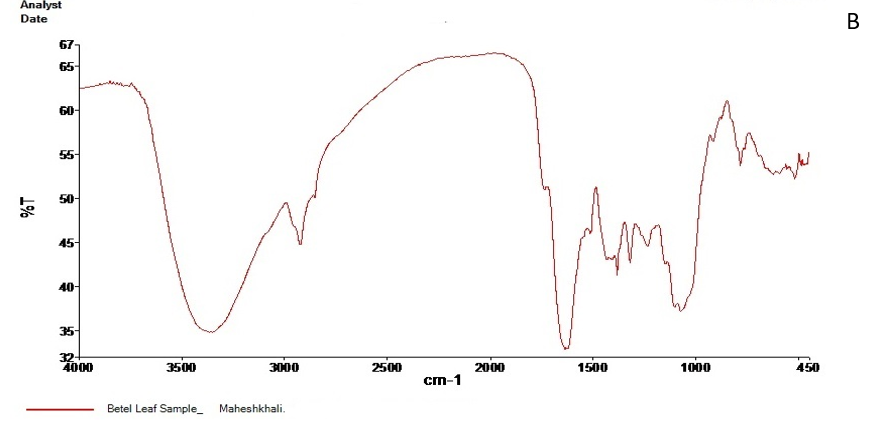
Figure 3. FT-IR spectrum of Barguna (A) and Moheshkhali (B) areas P. betle leave extracts
Table 3. FT-IR spectral peak values and functional groups obtained of the P. betle leave extracts collected from Barguna.
| Serial no. | Peak values | Functional groups |
|---|---|---|
|
|
3368.98 | -OH (Strong and broad band) |
|
|
2924.28 | C-H stretching of aliphatic alkanes CH2 and CH |
|
|
2853 | |
|
|
1740.67 | C=O |
|
|
1615.45 | Weak band |
|
|
1514.66 | C=C stretching of alkenes (Medium weak band) |
|
|
1433.66 | CH3 bending band |
|
|
1318.28 | |
|
|
1235.56 | C-O stretching |
|
|
1151.04 | |
|
|
1077.06 | |
|
|
916.63 | =C-H of alkenes |
|
|
781.42 | C-H stretching of aromatic ring |
Table 4. FT-IR spectral peak values and functional groups obtained for the P. betle leave extracts collected from Moheshkhali.
| Serial No. | Peak values | Functional groups |
|
|
3388.08 | -OH (Strong and broad band) |
|
|
2928.31 | C-H stretching of aliphatic alkanes CH2 and CH |
|
|
1838.22 | C=O |
|
|
1384.73 | CH3 bending band |
|
|
1320.28 | |
|
|
1238.38 | C-O stretching |
|
|
1073.81 | |
|
|
782.75 | C-H stretching of aromatic ring |
| 9. | 624.97 |
From last few decades, natural phytochemicals are in a great demand owing to their comprehensive biological active properties against a large number of diseases (18). The extracts of betel leaves showed a wide array of antibacterial, antifungal, antioxidant, and antihaemolytic activities in previous studies (6,19). In the present study, comparative evaluation of ethanol and acetone extracts of Barguna and Moheshkhali betel leaves were performed in light of their phytochemical profiling, antibacterial activity against three bacterial strains, MIC, and FT- IR profiling.
We did not find any antibacterial activity in the solvent control against all tested organisms. The ZOI of the extracts against selected bacterial strains were compared with the commonly used antibiotic, ciprofloxacin (30μg/disc) as standard, and were found to have significant ZOI against those strains. Ethanol extracts of both Barguna and Moheshkhali betel leaves showed 16 and 14.2 mm ZOIs, respectively against E. coli 25922 that is comparable with previous work where ethanol extract of betel leaves showed 14.67±1.15 mm ZOI against the same culture (14). However, acetone extracts of Barguna and Moheshkhali leaves showed 15.7 and 18 mm ZOIs, respectively, where the control drug ciprofloxacin showed 18.5 mm ZOI against the same bacterial strain. Ethanolic extract of Barguna and Moheshkhali betel leaves showed 20 and 22 mm ZOIs; respectively against S. aureus but acetone extract showed 18.7 and 20.2 mm ZOI, respectively, where ciprofloxacin showed 23 mm ZOI. But, previous studies with betel leaves showed 14.67 mm ZOI (14) and 18 mm ZOI (20) against S. aureus. However, highest ZOI was observed against B. cereus, irrespective of the origin of betel leaves and type of solvents used for extraction. We found the most significant antibacterial activity against B. cereus followed by S. aureus and E. coli.
Broth dilution method was performed to evaluate the MIC of Barguna and Moheshkhali betel leave extracts in acetone and ethanol. However, the value ranged from 2.12 to 8.5 mg/mL against the selected bacterial cultures. A recent investigation in 2017 showed bactericidal values of methanol, ethanol and aqueous extracts of P. betle leaves in the range of 1.125 to 2.25 mg/mL (12) where a research by Mahfuzul Hoque showed MIC values ranged from 0.625 to 0.75 mg/mL (14). The present study revealed the presence of steroids, tannin, glycosides, phenols, alkaloids, and saponin which were also found in previous studies (21,22). Both Barguna and Moheshkhali leaves showed similar results for the qualitative phytochemical screening except for flavonoid by Zn dust test, carbohydrate, and saponin by foam test. Interestingly, they were found in Barguna leaves only. The functional groups present in the extracts of the Barguna and Moheshkhali betel leaves were based on the absorption bands obtained at certain wave numbers by IR spectrophotometer analysis. A few differences were observed in the spectra of the extracts from both areas although they showed the mostly comparable result with previous work. Previously studied betel leave extracts were found to have functional groups as Hydroxy Chavicol, Chavibetol, and Eugenol that were reported to have antioxidant, anti-inflammatory, anti-platelet, antithrombotic, anti-bacteria and anti-fungal activities as well (19,23).
Our results showed comparative attribute between Barguna and Moheshkhali betel leave extracts prepared with acetone and ethanol. They showed mostly similar results on the properties of phytochemicals, antibacterial activities, and the presence of functional groups. They can be used in various purposes to increase the shelf life of foods or mitigating infection or improve health immunity etc. As they are cultivated in large amount in some countries, therefore, they can be appropriately used for various purposes following detailed studies.
We thank the Department of Microbiology, Primeasia University, Bangladesh for logistic support throughout our study.
Authors declared no conflict of interests.
Article Gallery
Received: 2020/02/3 | Accepted: 2020/03/1 | ePublished: 2020/04/9
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |











