BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-952-en.html
2- M.Sc of Microbiology, Medical Biotechnology Research Center, Ashkezar Branch, Islamic Azad University, Ashkezar, Yazd, Iran
.
Tosocomial infection or a healthcare-associated infection (HAI) is known as one of the serious challenges posed to the global health care
The outbreak of food-borne pathogens such as Escherichia coli, Clostridium botulinum, Bacillus cereus, Staphylococcus aureus, Salmonella spp, and Listeria monocytogenes in food safety have attracted the public attention to the need for developing new antimicrobial materials in order to ensure food safety and extend its shelf- life (1, 2). Directly add antimicrobial agents to food or packaging materials during the food production processes a convenient method for controlling microbial contamination in food and increasing the shelf life of foods (3). Recently, inorganic antimicrobial materials, such as metal oxides, have been applied in various areas for controlling microbes (4, 7, 8, 9-11). The synergistic effect of antimicrobials reduces the number of antimicrobials needed in foods and reduces side effects (20, 21).
The following bacterial strains used in this study: Escherichia coli PTCC1394 and Staphylococcus aureus PTCC1431 were purchased from Iranian microbial collection. CuO and MgO nanoparticles were purchased from SIGMA-ALDRICH and US Research Nanomaterials, respectively.
The agar diffusion method was used to investigate the antimicrobial properties of CuO and MgO nanoparticles. Antibacterial properties of CuO and MgO nanoparticle was examined by placing 20 μl of the CuO and MgO nanoparticle solution (0, 0.25, 0.5, 0.75, 1 and 1.5 mg/ml) alone or in combination with each other on TSA medium inoculated with 107cell/ml E. coli and S. aureus, respectively. Each concentration of CuO and MgO nanoparticle solution was placed on surface-inoculated TSA agars and incubated at 37 ◦C for 24h. The inhibition zone around each sample was applied to represent the antibacterial activity of each CuO and MgO nanoparticle’s concentration (22).
To determine the antimicrobial property of CuO and MgO nanoparticles, the liquid Tryptic Soy Broth culture (TSB), which contained (0, 0.5, 1 and 1.5 mg/ml) CuO and MgO nanoparticle solution alone or in combination with each other was inoculated with 107 cells/ml of E. coli and S. aureus, respectively. The samples were incubated at 37 ºC, and every three h for up to 24 h are examined by colony count method, respectively (23).
The fruit juice containing CuO and MgO nanoparticle solution (0, 0.5, and 1.5 mg/ml) alone or in combination with each other were prepared, respectively. The samples were inoculated with a mixture of S. aureus, and E. coli (107 cells/ml). The samples were incubated at 25 ºC, and bacterial counts were determined at 3, 8, and 24 h by colony count method, respectively (24).
Scanning electron microscopy was also used to show morphological changes of S. aureus, and E. coli after treatment with nanoparticles (24).
Statistical analysis
Data were analyzed by SPSS software version 16. Analysis of variance, and Duncan’s multiple range tests were applied to specify the significant difference of mean values. Unless stated otherwise, significance was expressed at 5% level.
Antibacterial properties of CuO and MgO nanoparticle solution (0, 0.25, 0.5, 0.75, 1, and 1.5 mg/ml) alone or in combination with each other were measured using the inhibition zone method against E. coli and S. aureus. The results of the inhibition zone of different concentrations of CuO and MgO nanoparticle alone or in combination with each other had no inhibition zone on E. coli and S. aureus (P>0.05).
In this research, CuO and MgO NP treatments showed a synergistic effect, and they were effective in reducing the number of E. coli and S. aureus in the medium and the fruit juices. In other researches (reference), the combination of nanoparticles was synergistic and reduced the number of bacteria, which is following our findings. Figure 1 and 2 show the effect of CuO and MgO nanoparticle treatment on the growth of E. coli and S. aureus in TSB broth at 37 ◦C. Treatments with the CuO and MgO nanoparticle in combination with each other had a significant inhibitory effect on the growth of E. coli and S. aureus during 24h of incubation, compared to the control. Among the ten concentrations of CuO and MgO nanoparticles, the treatment with 0/5 CuO + 1/5 MgO nanoparticle was the most effective one for E. coli (P<0.05) and S. aureus (P>0.05).

Figure 1. Effect of MgO and CuO Nanoparticles on E. coli in TSB (pvalue=0.00)

Figure 2. Effect of MgO and CuO Nanoparticles on S. aureus in TSB (pvalue=0.159)
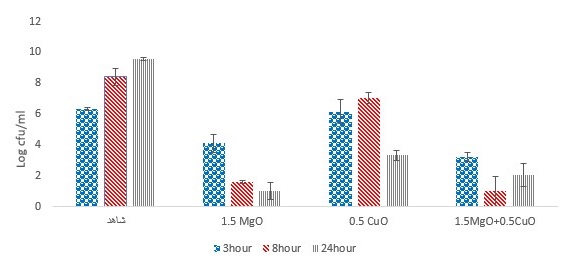
Figure 3. Investigation of the effect of MgO and CuO nanoparticles on E. coli bacteria in mango juice (pvalue=0.039).

Figure 4. Investigation of the effect of MgO and CuO nanoparticles on E. coli bacteria in pomegranate juice (pvalue=0.642).

Figure 5. Investigation of the effect of MgO and CuO nanoparticles on E. coli bacteria in peach juice (pvalue=0.607).
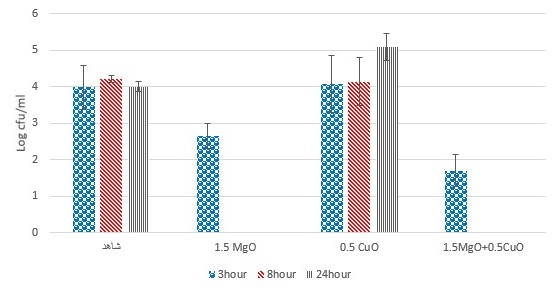
Figure 6. Investigation of the effect of MgO and CuO nanoparticles on S. aureus bacteria in mango juice (pvalue=0.278).

Figure 7. Investigation of the effect of MgO and CuO nanoparticles on S. aureus bacteria in pomegranate juice (pvalue=0.962).

Figure 8. Investigation of the effect of MgO and CuO nanoparticles on S. aureus bacteria in peach juice (pvalue=0.779).

Figure. 9. SEM image of E. coli treatments with MgO and CuO nanoparticles. Bacteria were incubated with a: TSB alone (control), b: CuO NPs, c: MgO NPs, d:MgO / CuO NPs.
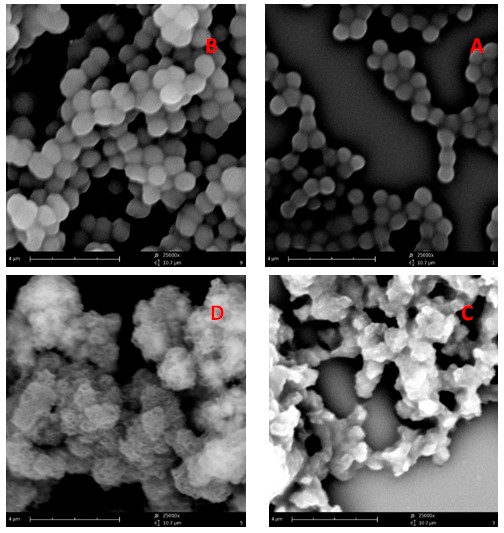
Figure. 10. SEM image of S. aureus treatments with MgO and CuO nanoparticles. Bacteria were incubated with a: TSB alone (control), b: CuO NPs, c: MgO NPs, d:MgO / CuO NPs.
The CuO and MgO nanoparticle were used as antimicrobial treatments in the fruit juices (mango, pomegranate, and peach). Effect of CuO and MgO nanoparticle on the growth of E. coli in fruit juice during 24 h of storage at 25 ◦C is demonstrated in figures 3, 4, and 5. Results showed that CuO and MgO nanoparticles could significantly inhibit or reduce E. coli in mango juice (P<0.05), pomegranate and peach juices (P>0.05).
Moreover, figures 6, 7, and 8 demonstrates that the antibacterial effect of MgO and CuO nanoparticles nanoparticle on S. aureus. Results showed that CuO and MgO nanoparticles could significantly inhibit or reduce S. aureus in mango juice, pomegranate, and peach juices (P>0.05). These findings implied that the treatment efficacy was more similar to the one obtained by MgO and CuO nanoparticle in the first set of experiments (Fig.2 and 3).
Based on the information available today, nanomaterials applied in the food industry include both inorganic and organic materials. Zinc oxide (ZnO) quantum dots, for example, has been used as an antimicrobial agent in liquid egg white samples (30). Similar inhibitory effects were observed for ZnO NP on the reduction of S. aureus and E. coli in milk samples. (24).
Jin and He (2011) examined the antibacterial activity of MgO NP alone or in combination with nisin or ZnO NP on E. coli O157: H7 and Salmonella Stanley. The results showed that the antimicrobial activity of MgO NP was vigorous. MgO NP showed a synergistic effect in combination with nisin. As well as in combination with the ZnO nanoparticles, their antibacterial activity is not synergistic against both pathogens (31).
SEM analysis of E. coli and S. aureus bacteria with and without nanoparticles treatment was performed to obtain definitive evidence of antimicrobial activity of the MgO and CuO nanoparticles. Figures 9 and 10 show electron microscopy of untreated E. coli and S. aureus (Fig. 9 and 10a), CuO treatment (Fig.9 and 10 b), MgO treatment (Fig. 9 and 10c), and MgO / CuO treatment (Fig. 9 and 10d) for 18 h in TSB.
As a control, untreated E. coli and S. aureus show rods and spheres shape in standard size, respectively, with cells whose structure is intact (Fig. 9, and 10a). Treatment with CuO nanoparticles reduces the number of E. coli bacteria and causes deformation on the cell surface and also creates troughs, but S. aureus in the vicinity of copper oxide nanoparticles has made the bacteria more adherent. (Fig. 9, and 10b). Treatment of bacteria with MgO NP causes severe damage to E. coli and S. aureus cells and resulting in the formation of cells with an irregular surface and change of membrane integrity (Fig. 9 and 10c). The combination of MgO / CuO has contracted cells and caused cell disintegration compared to healthy cells. These findings implied that the treatment efficacy was more similar to the one obtained by MgO and CuO nanoparticle in the first set of experiments.
Conclusion
The The results showed that the combination of CuO and MgO nanoparticles had synergistic behavior and had a significant effect on the growth control of S. aureus and E. coli in liquid media and fruit juices. New formulations of CuO and MgO nanoparticles can be effectively applied to control infection in the future. Also, making new compounds from these nanoparticles could have a protective role in the food industry and reducing pasteurization temperatures.
Acknowledgements
The authors gratefully acknowledge the generous cooperation of the Nano Structured Coatings Institute, Yazd Payame Noor University, Yazd, Iran.
Conflicts of Interest
This article is the result of an independent study conducted without organizational financial support. In the present study, the authors showed no conflict of interest.
Received: 2019/08/7 | Accepted: 2020/01/19 | ePublished: 2020/01/20
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








