BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1850-en.html
2- Department of Microbiology, University of Dhaka, Dhaka, Bangladesh , drmdhoque2004@yahoo.com
Food safety is a public health priority with paramount importance worldwide. Yearly, almost 1 in 10 people develops foodborne illness and 4, 20,000 die worldwide (1). The main reason behind food spoilage is the occurrence of different pathogenic microorganisms (bacteria, viruses, parasites, yeasts and molds). Ever-expanding antibiotics resistance among these pathogens are raising concern among food safety professionals and public health sectors due to a lack of innovation in the development of new antibiotics (2). Finding out a novel solution with next generation antimicrobials has become a public health emergency. Though chemical preservatives can prevent foodborne diseases, their continued application causes bio-accumulation of chemical residues in the food and feed chain, increasing microbial resistance to the chemicals used, and renders negative health impacts. An eco-friendly and chemical or antibiotic-free food preservation approach is the top demand from consumers to minimize food poisoning outbreaks caused by foodborne pathogens and spoilage bacteria. To meet up this demand, researchers are struggling for many years to develop novel antimicrobials and food preservation technologies. But very few of these are devoid of harmful chemicals. Thus the interest of using new preservation techniques with a natural or “green” image is increasing day by day. One such possibility is the use of essential oils (EOs) as food preservatives to destroy harmful pathogens in food. Essential oils are secondary metabolites of plants that play critical role in plants defense mechanism (3). Essential oils or their components have been demonstrated to not only possess broad-spectrum antibacterial properties (4, 5), but also antiparasitic (6), insecticidal (7), antiviral, antifungal, and antioxidant properties (8, 9). Although food industries primarily use essential oils as flavorings, they represent an intriguing source of natural antimicrobials for food preservation.
Cinnamon is one of the world’s oldest and most frequently consumed spice that is obtained from the inner bark of several tree species from the genus Cinnamomum. Ayurveda (the Indian system of medicine) has documented the therapeutic benefits of cinnamon for over 6000 years (10). Cinnamon contains various volatile components of multiple classes, namely monoterpenes, sesquiterpenes and phenylpropenes. Major active ingredient of cinnamon bark oil is cinnamalydehyde (precisely trans-cinnamaldehyde or 3-phenyl-2- propenal) (11) which is available commercially. There is a large bunch of previous report regarding the antibacterial, antifungal and antiviral activity of cinnamon essential oil and cinnamaldehyde (12-17) which renders the possibility for future formulation of organic food preservative using this agent. Cinnamon, according to Vasconcelos et al., can damage bacterial cell membranes, alter the lipid profile, inhibit ATPases, cell division, membrane porins, motility, and biofilm formation, and plays a role in anti-quorum sensing actions (18). Many prior in vitro studies also validated the ability of cinnamon essential oil and cinnamaldehyde in inactivating diverse bacterial populations in various food stuffs, also at different storage conditions and periods (19-23).
Despite of its reported advantageous features, commercial cinnamaldehyde is highly expensive, thus limiting its mass application. In contrast, extraction of cinnamon essential oil is more cost effective. To enrich the scientific community with more understanding regarding the comparative potentiality of cinnamon essential oil and cinnamaldehyde as a natural antimicrobial in food preservation, here we assessed the antibacterial efficacy of these agents against several commonly encountered food borne pathogens. We performed an in depth assessment to ensure that the agents are effective at various temperatures and pH, also show antibacterial activity against multi drug resistant (MDR) strains. As Salmonella typhimurium and Listeria monocytogenes are the two major causes of food borne illness worldwide (24-27), we showed CN and CEO mediated inactivation of these bacteria in artificially inoculated ground chicken meat to validate their applicability for the future formulation of a natural food preservative.
2.1 Test Organisms
Sixteen bacterial species (10 gram positive and 6 gram negative) and another 8 multidrug resistant Pseudomonas spp. isolates were used in the current study based on their availability and importance as foodborne pathogens. Supplementary Table 1 enlists the list of test organisms and the name of repositories from where the strains were collected. The collected strains were stocked at -80℃ with 20% glycerol in cryogenic vials. Tryptic Soy Agar (TSA) slants (Nissui, Japan) were used to prepare working cultures and stored at 4℃. The cultures were periodically sub-cultured to fresh TSA slants to ensure cell viability.
2.2 Extraction of Cinnamon Essential Oil
The solvent-solvent extraction method was used to extract cinnamon essential oil (CEO). The method was adopted from (20). Cinnamon barks were purchased from Dhaka new market, Bangladesh. Twenty grams of washed, dried and grounded barks were soaked in 80 mL n-hexane (Nacalai tesque Inc. Tokyo, Japan) and kept in shaker at room temperature for 48 hours. After that the mixture was squeezed with sterilized cheesecloth to separate the n-hexane fraction. The hexane from the fraction was evaporated at 400C using a drier, which left mass of organics called concrete. The remaining concrete was then dissolved in double amount of ethanol (95%) in a separation funnel and vigorously shaken for several hours to sediment the ethanol insoluble part. Finally, existing ethanol was removed by rotary evaporator and the mixture was filtrated by Whatman Filter Paper (Grade 1) to remove any remaining debris, leaving the absolute essential oil.
2.3 Screening the Antibacterial Activity of CEO and CN
The antibacterial activities of the extracted CEO and commercial CN (purchased from Nacalai Tesque Co, Kyoto, Japan) were evaluated using Kirby Bauer disc-diffusion assay against the selected food-borne pathogens and MDR isolates (Supplementary Table 1). From the 100% concentrated stock solutions of CEO and CN, 5% working solutions were prepared using 99% ethanol as solvent. Filter paper discs of six mm diameter were impregnated with 50 µL of 5% working solutions and dried overnight in hot air oven at 40°C to evaporate the solvent properly and applied on Mueller-Hinton Agar (MHA) plates inoculated with the test organisms. Zone of inhibition (ZOI) in bacterial growth were measured in mm (including 6 mm disc) after 24hrs of incubation at 37°C. A reference antibiotic, ciprofloxacin (5 µg/disc) was used as a positive control and disc containing ethanol only was used as a negative control.
Supplementary Table 1. List of test organisms used in this study.
| Foodborne pathogens and spoilage bacteria | |
| Test organisms | Source |
| Escherichia coli Escherichia coli DH5α Salmonella typhi Pseudomonas aeruginosa Klebsiella pneumoniae S. aureus B. cereus Listeria grayi |
Centre for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh. |
| Escherichia coli O157:H7 ATCC 12079 Salmonella typhimurium (ALM 40) Vibrio cholerae Vibrio parahaemolyticus ATCC 17802 Vibrio metschnikovii Listerrria monocytogenes S.aureus ATCC 25923 S. epidermidis |
International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) |
| Multidrug resistant (MDR) Pseudomonas spp. isolates | |
| Test isolates | Source |
| DMC-8b, BIHS-5, DMC-6, DMC-7, BIHS-7, DMC-24, BIHS-8, DMC-20b | Microbial Genetics and Bioinformatics Laboratory (MGBL), University of Dhaka, Bangladesh. |
2.4 Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of CEO and CN
MIC and MBC of CEO and CN were determined by broth microdilution method followed by culturing on MHA, as described previously (21). MIC was defined as the lowest concentration of CEO and CN at which no visible growth of the tested organisms were demonstrated on MHB after 24hrs incubation. Minimum bactericidal concentration (MBC) was defined as the lowest concentration that yielded no or only single colony in the MHA after another 24hrs incubation.
2.5 Determination of the Effect of Heat Treatment and pH Alteration on the Antimicrobial Activity of CEO
5% solutions of CEO were incubated at 37°C, 50°C, 75°C and 100°C temperatures for 30 minutes and cooled down. Another set of 5% solutions were adjusted to pH 2.0, pH 7.0 and pH 9.0 with 50mM Phosphate buffer followed by filtration with a membrane filter (0.45 μm). After that the antibacterial activity of heat treated and pH adjusted CEO were determined by disc diffusion method.
2.6 Time Killing Assay
CEO and CN were evaluated to investigate their kinetic destruction pattern against Salmonella typhimurium (ALM 40) and Listeria monocytogenes, two major causative agent of foodborne illness. Bacterial cells were grown on MHB for 6 hrs., followed by centrifugation and re-suspension in normal saline. 1ml of bacterial suspension was then transferred to MHB media containing 1xMIC concentration of CEO or CN. Finally, the bacterial concentration in the mixture was adjusted to 107 CFU/mL. To determine cell viability and bacterial growth in comparison with control (no plant extract), the prepared mixtures were inoculated on the TSA plates at predetermined time intervals (0h, 1h, 3h, 5h, 7h, 9h) and incubated for 24hrs at 370C. Number of viable cells were counted in log CFU/mL after incubation which indicated the kinetic destruction pattern of CEO and CN.
2.7 Determination of CN and CEO Mediated Inactivation Dynamics of S. typhimurium (ALM 40) and L. monocytogenes in Ground Chicken Meat
Inactivation dynamics of S. typhimurium (ALM 40) and L. monocytogenes in ground chicken meat at different storage conditions were determined by a slight modification described by Hoque et al. (21) Nalidixic acid resistant S. typhimurium (ALM 40) was inoculated into 200gm ground chicken meat to obtain a final concentration of 8.85 or 8.44 log CFU/gm. L. monocytogenes at a final concentration of 7.73 or 7.35 log CFU/gm was used to inoculate in ground chicken meat. Inoculated meat samples were uniformly mixed with a sterilized spoon. 3xMIC and 5xMIC concentrations of CN and CEO were then added with the S. typhimurium (ALM 40) and L. monocytogenes inoculated meat samples, respectively. After proper mixing, meat samples were divided in small aliquots (5 g each) and packed in UV radiated Ziplock packs and stored at 4°C and -20°C for 14 days. Control samples with no CEO/CN treatment were also stored similarly. Microbiological analysis of control and treated sample was done on 1-14 days of post storage. Using a homogenizer, each samples were homogenized in sterilized peptone water for 3 minutes. The mixtures were diluted using tenfold serial dilutions (10-7) and 0.1ml of each diluent were surface plated on Tryptone Soy Agar containing 50 μg/mL nalidixic acid (TSAN), which is a non-selective general purpose media; Bismuth Sulfite Agar containing 50 μg/mL nalidixic acid (BSAN) and Listeria Selective Agar containing 50 μg/mL nalidixic acid (LSAN) which are the selective media for Salmonella spp and Listeria spp, respectively. The plates were then incubated at 37°C for 24 hours and then total viable cell was counted in both non-selective and selective media.
2.8 Statistical Analysis
ZOI were represented as means ± SD where SD means the standard deviation of the values obtained from three to four individual experiments. GraphPad Prism software was used to evaluate the significance among different data by analysis of variance (ANOVA). 5% level of significance was used to denote significant differences in the data.
3.1 Antibacterial Effects of Extracted Cinnamon Essential Oil (CEO) and Commercial Cinnamaldehyde (CN)
CEO and CN were applied to 16 test organisms and 8 multidrug resistant isolates (Supplementary Table 1). All the test organisms showed positive response to CEO and CN except E. coli 0157:H7 to CEO (Table 1). ZOI ranged from 10.0 mm to 12.0 mm for 5% v/v CEO and 13.0 mm to 20.0 mm for 5% v/v CN. CEO and CN showed highest antibacterial activity against V. metschnikovii and B. cereus, providing a ZOI of 12 mm and 20mm respectively. The lowest CN activity was observed against E. coli (13 mm ZOI). E. coli O157:H7 withstood the antibacterial effect of CEO (no inhibition), but was highly affected by CN (14 mm ZOI). The standard antibiotic (CIP 5μg/disc) used in this study showed higher activity than both CEO and CN, reflecting that higher concentration (>5%) of CN and CEO will be required to achieve similar effect of the standard antibiotic. Although the antibacterial effect of CN was found to be higher than CEO, it is noteworthy that the difference was very low in case of ten test strains (E. coli, E. coli DH5α, S. typhi, S. typhimurium (ALM 40), V. cholerae, V. metschnikovii and K. pneumoniae, S. aureus, L. monocytogenes and L. grayi), differing from only 3-5mm ZOI. Also, the ZOI of CN against V. parahaemolyticus, K. pneumoniae and B. cereus were found to be very close to that of the standard antibiotic, differing from only 2-3mm. All these findings summarize a satisfactorily good level of antimicrobial activity of extracted cinnamon essential oil and its active ingredient cinnamaldehyde, even at 5% v/v concentration only.
Noteworthy, CN showed positive response against all the multidrug resistant isolates and provided 17 to 34 mm inhibition zone (Table 1). It was evident that CN showed higher antibacterial activity to MDR Pseudomonas spp. strains in comparison with sensitive P. aeruginosa. The zone diameter in these cases differed from 16mm in case of sensitive one to (17-34 mm) in case of MDR isolates. This gives an insight that possibly the MDR isolates get more sensitive to the natural antibacterial product cinnamaldehyde due to acquiring antibiotic resistance. Also, the extracted CEO was found to be more active against MDR Pseudomonas spp. isolate DMC-8b (19 mm ZOI) than sensitive P. aeruginosa (10 mm ZOI). Although our extracted CEO did not show sufficient antibacterial activity against six tested strains at 5% v/v concentration, positive antibacterial activity against DMC-8b and BIHS-8 provides an indication that the agent might be effective against MDR isolates at higher concentration.
3.3 Minimum Inhibitory Concentration of CEO and CN
MIC values of CEO against the test organisms ranged from 5% to 0.625% v/v (Table 2) which ensures that a satisfactorily low concentration of this agent is capable of inhibiting the growth of food borne pathogens. CN showed much more promising result compared to CEO. The MIC value of CN ranged from 0.078% to 0.3125% v/v which indicates a very desirable antibacterial activity. V. cholerae, B. cereus and L. grayi, showed a CN MIC value of 0.078% which is an up to scratch finding. Minimum Bactericidal Concentration (MBC) values of CEO and CN was found to be two fold lower than the respective MIC values in all the tested cases. The value ranged from 1.25%-10% v/v in case of extracted CEO and 0.3125%-0.625%-v/v in case of commercial CN (Table 2).
Table 1. Antibacterial activities of extracted cinnamon essential oil (CEO) and commercial cinnamaldehyde (CN) against foodborne pathogens and spoilage bacteria.
| Organisms | Zones of inhibition (in mm) | ||
| CEO (5% v/v) | CN (5% v/v) | CIP (5μg/disc) | |
| Escherichia coli | 10 ± 0.098 | 13 ± 0.56 | 22 ± 1.44 |
| Escherichia coli O157:H7 | 6 ± 0.0 | 14 ± 0.28 | 22.5 ± 0.28 |
| Escherichia coli DH5α | 11 ± 0.5 | 15 ± 0.57 | 22 ± 0.5 |
| Salmonella typhi | 11 ± 0.763 | 15 ± 0.157 | 24 ± 1.0 |
| Salmonella typhimurium (ALM 40) | 10 ± 0.57 | 15 ± 0.763 | 24.5 ± 0.5 |
| Vibrio cholerae | 10 ± 0.5 | 14 ± 0.28 | 19.5 ± 0.5 |
| Vibrio parahemolyticus | 10 ± 0.57 | 16 ± 0.57 | 18 ± 0.28 |
| Vibrio metschnikovii | 12 ± 0.28 | 17 ± 0.57 | 23 ± 0.28 |
| Pseudomonas aeruginosa | 10 ± 0.5 | 16 ± 0.76 | 26.5 ± 0.5 |
| Klebsiellla pneumoniae | 11 ± 0.28 | 15 ± 0.57 | 18 ± 0.5 |
| Bacillus cereus | 11 ± 0.28 | 20 ± 0.28 | 19.5 ± 0.5 |
| Staphylococcus aureus | 10 ± 0.00 | 15 ± 0.5 | 22 ± 0.28 |
| Staphylococcus aureus ATCC-25923 | 11 ± 0.28 | 17 ± 0.76 | 22.5 ± 0.5 |
| Staphylococcus epidermidis | 10 ± 0.28 | 16 ± 0.28 | 23 ± 0.57 |
| Listeria monocytogenes | 10 ± 0.28 | 14 ± 0.763 | 23 ± 0.56 |
| Listeria grayi | 12 ± 0.57 | 16 ± 0.28 | 27 ± 0.57 |
| DMC-8b | 19 ± 0.13 | 34 ± 0.047 | 0 |
| BIHS-5 | 0 | 18 ± 0.23 | 0 |
| DMC-6 | 0 | 17 ± 0.047 | 0 |
| DMC-7 | 0 | 20 ± 0.047 | 0 |
| BIHS-7 | 0 | 19 ± 0.094 | 0 |
| DMC-24 | 0 | 17 ± 0.4 | 0 |
| BIHS-8 | 10 ± 0.205 | 21 ± 0.082 | 0 |
| DMC-20b | 0 | 17 ± 0.5 | 0 |
The mean diameter of zone of inhibition including the diameter of the disc (6 mm) has been represented as (Mean ± Standard deviation) mm (n=3); P<0.05; CIP=Standard antibiotic ciprofloxacin (5μg/disc).
Table 2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values of extracted cinnamon essential oil (CEO) and commercial cinnamaldehyde (CN).
| Organisms | CEO | CN | ||
| MIC (% v/v) |
MBC (% v/v) |
MIC (% v/v) |
MBC (% v/v) |
|
| Escherichia coli | 5 | 10 | 0.3125 | 0.625 |
| Escherichia coli O157:H7 | 5 | 10 | 0.3125 | 0.625 |
| Escherichia coli DH5α | 2.5 | 5 | 0.3125 | 0.625 |
| Salmonella typhi | 5 | 10 | 0.3125 | 0.625 |
| Salmonella typhimurium (ALM 40) | 5 | 10 | 0.3125 | 0.625 |
| Vibrio cholerae | 2.5 | 5 | 0.078 | 0.3125 |
| Vibrio parahemolyticus | 5 | 10 | 0.3125 | 0.625 |
| Vibrio metschnikovii | 0.625 | 1.25 | 0.3125 | 0.625 |
| Pseudomonas aeruginosa | 1.25 | 2.5 | 0.3125 | 0.625 |
| Klebsiellla pneumoniae | 5 | 10 | 0.3125 | 0.625 |
| Bacillus cereus | 1.25 | 2.5 | 0.078 | 0.3125 |
| Staphylococcus aureus | 5 | 10 | 0.3125 | 0.625 |
| Staphylococcus aureus ATCC-25923 | 2.5 | 5 | 0.3125 | 0.625 |
| Staphylococcus epidermidis | 5 | 10 | 0.3125 | 0.625 |
| Listeria monocytogenes | 2.5 | 5 | 0.3125 | 0.625 |
| Listeria grayi | 1.25 | 2.5 | 0.078 | 0.3125 |
3.4 Effect of heat treatment and pH alteration on the antibacterial activity of CEO
CEO showed positive response after being treated with all the tested temperatures (370 C, 500 C, 750 C, 1000 C) suggesting that it’s activity was not destroyed at high temperatures even after 30 min treatment. An interesting finding was that the antibacterial effect of CEO against all the test strains increased with increasing temperature. The ZOI against the test strains ranged from 9-14mm at 370 C, 10-15mm at 500 C, 10-16mm at 750 C and 18-24mm at 1000 C of heat treatment (Table 3). Here, it is remarkable that after 1000 C heat treatment, our extracted CEO showed significantly higher level of antibacterial effect than the commercial CN itself at 370C. The zones of inhibition provided by CEO at 5% v/v concentration after 1000 C heat treatment ranged from 18-24mm (Table 3), whereas the inhibition zone of CN at 5% concentration against the respective strains ranged from 13-15mm (Table 1).
Potential antibacterial activity of CEO was observed at all the tested pH condition (pH 2, 7 and 9) indicating a broad range of pH sustainability of this agent. Due to the alteration of pH, slight increase or decrease in the activity of CEO was noticed which also varied with different organisms. Highest activity was found in case of Salmonella typhi at pH 2 and pH 9. Lowest activity was observed against S. typhimurium (ALM 40) at pH 9. The ZOIs of CEO against six tested strains ranged from 17-19mm at pH 2, 14-19mm at pH 7 and 13-19mm at pH 9 (Table 3). Overall, acidic pH was found to be more favorable for exerting higher antibacterial effect, though the antibacterial activity was not diminished due to pH change.
3.5 Kinetic destruction pattern of CEO and CN
S. typhimurium (ALM 40) and L. monocytogenes were challenged with extracted CEO and commercial CN at 1xMIC concentration to investigate whether the growth inhibiting effect of these antimicrobial agents is attributable to bacteriostatic or bactericidal activity with required time course. Bacterial viability was checked at every one-hour time points during incubation and viable cells were counted. In the absence of the CEO and CN, viable cells of Salmonella typhi and Listeria monocytogenes increased from 6.07 to 13.21 log CFU/ml and 6.96 to 10.15 log CFU/ml, respectively after 6hrs of incubation (Fig. 1). Significant decrease in the viable cells were observed in the presence of CEO and CN. Corroborating to the previous findings, CN came up with more desirable outputs in comparison with CEO. Within 3hr of incubation of S. typhimurium (ALM 40) with CN, complete eradication of the viable cells (6.07 log CFU/ml reduction in viable count) was observed, whereas CEO required 6hrs to exert the complete bactericidal effect. CEO and CN reduced the viable count of L. monocytogenes (from 6.96 log CFU/ml to 0) within 4hrs and 3hrs of exposure, respectively. However, the killing rate was found to be exponential in both cases ensuring both CEO and CN to be bactericidal at 1xMIC concentration.
Table 3. Effect of heat treatment and pH alteration on the antibacterial activity of extracted cinnamon essential oil (CEO).
| Test organisms | Effect of Temperature (Zone of inhibition in mm) |
Effect of pH (Zone of inhibition in mm) |
|||||
| 370 C | 500 C | 750 C | 1000 C | pH 2 | pH 7 | pH 9 | |
| Escherichia coli | 12 ± 0.58 | 13 ± 0.2 | 13 ± 0.08 | 18 ± 0.58 | 17 ± 0.5 | 17 ± 0.67 | 15 ± 0.3 |
| Escherichia coli O157:H7 | ND | ND | ND | ND | 17 ± 0.2 | 14 ± 0.3 | 17 ± 0.1 |
| Salmonella typhi | 10 ±0.6 | 10 ± 0.6 | 10 ± 0.2 | 20 ± 0.2 | 19 ± 0.44 | 15 ± 0.1 | 19 ± 0.03 |
| Salmonella typhimurium (ALM 40) | 14 ± 0.08 | 15 ± 0.08 | 16 ± 0.58 | 24 ± 0.08 | 17 ± 0.4 | 18 ± 0.27 | 13 ± 0.15 |
| Vibrio cholerae | 9 ± 0.08 | 10 ± 0.58 | 11 ± 0.6 | 19 ± 0.58 | ND | ND | ND |
| Klebsiella pneumoniae | 9 ± 0.2 | 10 ± 0.2 | 12 ± 0.58 | 20 ± 0.08 | ND | ND | ND |
| Staphylococcus aureus | ND | ND | ND | ND | 18 | 19 | 19 |
| Listeria monocytogenes | 12 ± 0.58 | 14 ± 0.58 | 15± 0.08 | 22 ± 0.6 | 19 | 19 | 17 |
Mean diameter of zone of inhibition including the diameter of the disc (6 mm) has been represented as (Mean ± Standard deviation) mm (n=3); P < 0.05. ND: Not determined.

Figure 1. Kinetic destruction pattern of cinnamon essential oil (CEO) and cinnmaldehyde (CN) on the growth of Salmonella typhimurium (ALM 40) (A) and Listeria monocytogenes (B). 1xMIC concentration of the agents were applied to observe viable cells during incubation at 37 °C. The results are represented as log CFU/ml where the counts are mean values ± standard deviation of three independent experiments.
3.6 Inactivation dynamics of S. typhimurium (ALM 40) inoculated in ground chicken meat due to the treatment with CN
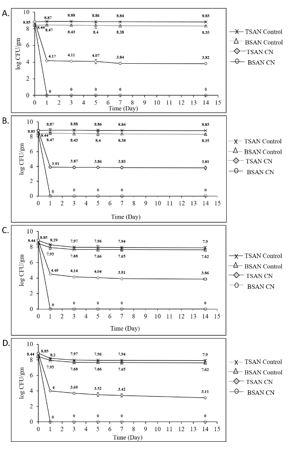
Salmonella typhimurium (ALM 40) inoculated ground chicken meat was challenged by 3xMIC and 5xMIC concentration of commercial CN to inactivate the organism in meat when stored at 4oC and -20oC for 14 days. After artificially inoculating the meat sample with the test organisms, the viable count was 8.85 log CFU/gm in TSAN and 8.44 log CFU/gm in BSAN (Fig. 2). The viable count decreased rapidly in both cases after treatment with CN. While stored at 40 C for one day, meat samples treated with 3xMIC concentration of CN showed a reduction of viable cell counts by 4.68 log CFU/gm in nonselective TSAN medium. 14 days storage of the sample in the same condition showed a decrease of viable count by 5.03 log CFU/gm in TSAN medium. After 14 days of storage in this condition, the final cell count was found 3.82 log CFU/gm in TSAN medium (Fig. 2A). However, after treatment with 5xMIC concentration of CN, S. typhimurium (ALM 40) count in TSAN decreased up to 4.94 log CFU/gm in one day while stored at 40 C and thereafter a continual slight reduction was noticed through the entire storage period. Finally, the cell count was noticed to be 3.81 log CFU/gm in TSAN medium (Fig. 2B). In contrary, the microbial count in untreated control samples were reduced only (0.02-0.09) log CFU/gm after 14 days of storage. Here, an interesting finding was that no viable cell was recovered in the selective BSAN medium inoculated with CN treated meat samples just after one day of storage at 40 C.
Figure 2. Inactivation dynamics of Salmonella typhimurium (ALM 40) in ground chicken meat treated with cinnamaldehyde (CN). A. 3xMIC concentration of CN and stored at 40C. B. 5xMIC concentration of CN and stored at 40C. C. 3xMIC concentration of CN and stored at -200 C. D. 5xMIC concentration of CN and stored at -200 C. The results are represented as log CFU/gm where the counts are mean values ± standard deviation of three independent experiments.
After one day of storage of the CN treated meat sample at -200 C, the viable count in TSAN medium reduced by 4.36 and 4.85 log CFU/gm due to the exposure of 3xMIC and 5xMIC concentration of CN, respectively (Fig. 2 C and 2D). After 14 days of exposure in the mentioned condition, viable cell count in TSAN was reduced to 3.86 and 3.11 log CFU/ml, in case of 3xMIC and 5xMIC concentration of CN, respectively. Complete eradication of the test organisms was observed in BSAN medium after 1 day of exposure with each concentration. In contrast, untreated control samples showed only a reduction of (0.82-0.95) log CFU/gm after the entire storage period.
The comparative study of the obtained results suggested that the activity of CN in meat sample is slightly dose-dependent. Microbial reduction by 3xMIC and 5xMIC concentration of CN differed only from 0.26 and 0.49 log CFU/gm in TSAN medium while stored at 40 C and -200 C, respectively (Fig. 2). But no difference between the efficacies of these two concentration was noticed while cultured the samples in selective BSAN medium. Both of the concentrations were able to cause great damage of the microbial cell and the cells could not recover while plated on BSAN medium, thus giving zero cell count in BSAN medium just after one day of storage. We also observed that storage condition does not affect much on the efficacy of CN. During storage at 40 C and -200 C, the viable cell reduction differed only (0.32-0.09) log CFU/gm. This insignificant difference can be explained by the slightly higher reduction of microbial cells in control samples while stored at -200 C. This finding suggest that food treated with CN can be stored equally good at both 40 C and -200 C refrigerator. There was rapid reduction of cell count in CN treated meat samples just after one day of storage and no further increase of the count was noticed when the storage period was extended to 14 days. The finding supports that prolonged storage period also does not hamper the efficacy of CN.
In this study, we used both nonselective TSAN and selective BSAN medium for the enumeration of S. typhimurium (ALM 40) on treated and untreated meat. Here, we found that, regardless of the CN concentration or storage condition, higher number of microbial population recovered on TSAN medium than on BSAN. Microbial counts ranged from (3.91-4.49) log CFU/gm higher due to the inoculation of treated meat samples in TSAN compared to inoculated on BSAN.
Overall, CN treated meat samples showed significant decrease in S. typhimurium (ALM 40) count in comparison with untreated one. Also, the viable count in selective BSAN medium reduced from 8.44 to 0 log CFU/ml just after one day of storage which ensures a highly reliable level of pathogen eradication in preserved meat.
3.7 Inactivation dynamics of L. monocytogenes inoculated in ground chicken meat due to the treatment with CEO
Ground chicken meat inoculated with L. monocytogenes was challenged by 3xMIC and 5xMIC concentration of extracted CEO and stored at 4oC and -20oC for 14 days. Initial viable count was 7.73 and 7.35 log CFU/gm in TSAN and LSAN, respectively (Fig. 3). Untreated samples exhibited a growth increase of 0.45 and 0.75 log CFU/gm during storage at 4oC and -20oC, respectively. One day of storage at 40C with 3xMIC and 5XMIC of CEO resulted in the reduction of 2.89-3.66 log CFU/gm viable count in TSAN medium (Fig. 3A and 3B). While stored at -200C, 3.14-3.62 log CFU/gm reduction was observed in TSAN due to 3xMIC and 5XMIC of CEO (Fig. 3C and 3D). 14-day storage at 40C reduced 4.73 and 4.23 log CFU/gm in TSAN due to 3xMIC and 5XMIC of CEO, while storage at -20oC reduced 3.93 and 4.1 log CFU/gm, respectively. Storage condition (40C and -200C) affected only slightly (0.23 to 0.13 log CFU/gm) to the recovery rate of microorganisms in TSAN. Inactivation of L. monocytgenes in meat sample was found to be slightly dose-dependent, although the difference (0.17-0.77 log CFU/gm) was non-significant. Prolonged storage did not diminish the antimicrobial potential of CEO. No viable growth of Listeria was observed in selective LSAN media due to CEO treatment just after one day of storage. Both 3xMIC and 5xMIC of CEO was equally effective against L. monocytogenes inoculated meat samples stored at 40C and -20oC which was confirmed by complete inhibition of Listeria growth in LSAN.
Figure 3. Inactivation dynamics of Listeria monocytogenes in ground chicken meat treated with cinnamon essential oil (CEO). A. 3xMIC concentration of CEO and stored at 40C. B. 5xMIC concentration of CEO and stored at 40C. C. 3xMIC concentration of CEO and stored at -200 C. D. 5xMIC concentration of CEO and stored at -200 C. The results are represented as log CFU/gm where the counts are mean values ± standard deviation of three independent experiments.
Food borne pathogens are a threatening medical concern around the world nowadays, calling for progressively powerful food preservation methodologies. Negative concerns of the consumers toward synthetic or chemical food preservatives boost the demand for an alternative natural antimicrobial which leads to the notion of utilizing essential oils as food preservative. Several previous studies have archived the antimicrobial properties of different essential oils (28, 29). The current study examined the antibacterial properties of extracted cinnamon essential oil (CEO) and commercial cinnamaldehyde (CN) against 16 food borne pathogens and 8 multidrug resistant Pseudomonas spp.
The results presented in Table 1 showed that both of the compounds under investigation exhibited significant antibacterial activity as evidenced by their zones of inhibition. Vibrio metschnikovii and Listeria grayi was found to be the most sensitive while E. coli and S. aureus to be the least. CN showed higher antibacterial activity than CEO against all the isolates possibly because of being a highly purified commercial product. Despite the fact that our extracted CEO did not undergo high level of purification like the commercial CN, still it demonstrated nearly equal antibacterial effect like CN (differing from only 3-5mm zone of inhibition) in case of ten test isolates (Table 1). These findings indicate that CEO can also offer expected level of antibacterial effect as its effectiveness is mostly attributed to its major ingredient cinnamaldehyde as previously stated (30). Although the standard antibiotic (CIP 5μg/disc) showed higher activity than 5% CEO and CN, it is prudent to employ this natural product in exchange of antibiotics to combat the ever increasing antibiotic resistance of pathogenic microbes, which is a global concern in this century. Moreover, any antibacterial formulation carrying plant essential oils is expected to be much cheaper than the highly expensive antibiotics. CN showed high level of antibacterial activity against all the MDR isolates used in our study. CEO also showed antibacterial effect against two MDR isolates. The findings corroborated with Utchariyakiat et al. stating cinnamon bark oil and cinnamaldehyde to have strong antibacterial potential against MDR-PA isolates with MIC of 0.0562–0.225 % v/v and MBC of 0.1125–1.8 % v/v (31). Moreover, they claimed that the combination therapy of cinnamon bark oil and cinnamaldehyde with colistin can be a beneficial alternative to antibiotic therapy. Noteworthy, growth inhibition of MDR Pseudomonas spp. by CN was higher than the sensitive P. aeruginosa (Table 1), which gives an insight that possibly the MDR isolates get more sensitive to the natural antibacterial product due to acquiring antibiotic resistance. Another previous finding reported that growth inhibition of MDR K. pneumoniae by natural compounds (extracts of Chamomile, Aquatic Pennyroyal and Nettle plants) was higher than seven different antibiotics (32).
Results of MIC determination were likewise consistent with the antibacterial sensitivity profile indicating CN to possess higher antibacterial activity than CEO. MIC value of CEO ranged from 0.625% to 5% v/v (MBC 1.25% to 10% v/v) and CN ranged from 0.078% to 0.3125% v/v (MBC 0.3125% to 0.625% v/v). Several earlier studies reported the MIC values of cinnamaldehyde and their extracted cinnamon EO against food borne pathogens. Hili and colleagues have extracted the essential oil from Cinnamomum zeylanicum leaf oil and assayed its antimicrobial activity against three bacteria and four yeast strains. Their results showed that the leaf oil completely inhibited the growth of E. coli, S. aureus, and P. aeruginosa at the level of 500 μg/mL which is consistent with our findings (33). Chang et al. showed that MIC of C. osmophloeum essential leaf oil was 500 μg/mL against K. pneumoniae; Salmonella spp. and 250 μg/mL against the other isolates (E. coli, P. aeruginosa, V. parahemolyticus, E. faecalis, S. aureus, S. epidermidis, and methicillin-resistant S. aureus) (34). Our extracted CEO had a MIC value of 0.625% to 5%v/v (78.8-630.36 μg/mL) against the test isolates, which was consistent with other previous studies reporting MICs of comparable ingredients ranging from 75 µg/mL to 600 µg/mL (30). This correspondence with earlier research confirms CEO's projected effectiveness against food-borne pathogens. CEO and CN both manifested similar bactericidal activity against both gram positive and gram negative microorganisms which indicates their broad spectrum applicability.
Heat treatment of CEO with various temperatures (370 C, 500 C, 750 C, 1000 C) for 30 min revealed that the antibacterial effect of CEO was not destroyed by increasing temperature, rather the activity increased (Table 3). Hoque et al. also described similar findings (21). Highest antimicrobial activity of cinnamon at 600C was reported previously, although the antibacterial activity was not fully diminished due to increased heat treatment (35). Strikingly, after 1000 C heat treatment, our extracted CEO showed significantly stronger antibacterial effect than the commercial CN itself (Table 3 and Table 1). This provides an indication of the heat induced activation of the active ingredient of cinnamon essential oil due to the destruction of interfering components present in extracted CEO. This finding implies that our extracted CEO (after 100°C heat treatment) can be a reasonable alternative to commercial cinnamaldehyde, thereby reducing the additional cost of extracting cinnamaldehyde commercially from cinnamon essential oil. Furthermore, due to CEO’s great temperature tolerance, the oil can retain its antimicrobial activity at high temperatures of cooking and so can be employed as potential preservatives for the foods processed at high temperatures. Similar to heat treatment, pH changes had no effect on CEO’s effectivenessand the activity was not diminished at various pH (pH 2, 7 and 9). In some cases, a little increase or decrease of activity was observed that also varied with the isolates. Various pH tolerance of CEO indicated the oil to be compatible for preserving both acidic and alkaline foods.
The results of time killing assay against S. typhimurium (ALM 40) showed that bacterial cells numbered 6.07 log CFU/mL were being killed within 6hrs and 3hrs after treatment with 1xMIC of CEO and CN, respectively. Senhaji et al. showed the kinetic destruction pattern of essential oil from Cinnamomum zeylanicum on the growth of E. coli O157:H7. At 0.05% concentration, the agent showed bactericidal effect after 30 min of incubation (36). Time-killing graphs demonstrated by Utchariyakiat et al. showed the reduction of viable cells of P. aeruginosa PAO1 approximately 6 log CFU/mL after being treated with cinnamaldehyde and cinnamon bark oil for 1.3 and 2 h, respectively (31). This difference in time killing graph obtained by our study and the mentioned others can be explained by the extensive antimicrobial resistance and heat tolerance of S. typhimurium 26, 37, 38) which also supports S. typhimurium to be capable of tolerating essential oil treatment for longer period of time than other bacteria. Kinetics of L. monocytogenes destruction was faster (3hrs and 4hrs due to 1xMIC of CN and CEO) than S. typhimurium. Paudel and co-workers also reported higher log reduction of L. monocytogenes than Salmonella spp. in melon model (39), thus reflecting the later one to be more antimicrobial resistant. 2x MIC and 4x MIC of ethanolic clove and negro pepper extracts was required to destruct 3 log units of Listeria spp. as obtained by previous study (29). Our finding validates cinnamon to be more effective against Listeria than clove and negro pepper. Nozohour et al. also supported that trans-cinnamaldehyde is more effective against Streptococcus equi than other essential oil main components (1, 8 Cineole and Pulegone) (40).
Being rich in essential nutrients, meat serves as a suitable source of microbial contamination as well as foodborne diseases. This is the reason to choose meat as our experimental model for studying bacterial inactivation dynamics using CEO and CN. Following the inactivation test, we observed that CEO and CN functioned in slightly dose-dependent manner which matches previous findings (23, 31). We also noticed that neither the storage condition nor the storage time had a significant impact on the activity of the antimicrobials, ensuring that the agents may be used at various storage conditions and also for a prolonged period of time. However, in all cases of the test, complete reduction of viable counts was observed just after 1 day of treatment with both 3xMIC and 5xMIC of CN while cultured on selective BSAN medium. In agreement with our study, Yossa et al. showed up to six log reductions in the viable count of S. typhimurium in 2% cinnamaldehyde treated organic soil (23) and Al-Nabulsi et al. reported reduction of 2.87, 2.64 or 2.35 log10 CFU/ml in Salmonella spp. count due to 2.0% Cinnamon oil treatment at tahini stored at 37, 25 or 100C, respectively (41). According to a prior study (20), crude cinnamon EOs at 2.5% and 5% were not shown to be effective against L. monocytogenes. However, when the concentration was raised to 7.5% and 12.5% (3xMIC and 5xMIC of CEO) in our study, we observed inactivation of this species. The mentioned study concluded that clove EOs to be superior to cinnamon EOs for Listeria inactivation in ground beef, while we report increased concentration of CEO and only minute concentration of CN to be highly effective in preserving ground chicken meat.
A discrepancy between the count in nonselective TSAN and selective BSAN medium was observed in our study which might be related to the recovery of some injured cells in the nonselective medium. But the bacterial count in TSAN were also reduced sufficiently just after 1 day and remained in static phase during the whole preservation period. Though the nonselective medium facilitated the growth of some injured cells, it is unlikely that these cells can endure the harsh environment of human stomach and gut. And thus, this injured cells should carry a little risk to human health. Pointedly, CEO and CN treated samples showed significantly higher reduction of microbial cells than untreated ones in all cases.
Multiple previous studies reported on the inactivation of food pathogenes using plant extracts and essential oils. 2.38 and 2.78 logarithmic reductions of S. typhimurium on tomatoes treated with 4% sumac extract and 100 ppm oregano (respectively) were reported by Gündüz et al. (42). The team of Vallejo et al. demonstrated that strawberry polyphenols can effectively inactivate Listeria monocytogenes and Salmonella typhimurium in strawberry juice (43). Tassou et al. showed that 1%, 1.5% and 2% v/w concentrations of mint (Mentha piperita) essential oil killed S. enteritidis in tzatziki after being stored at 4°C for 6, 4 and 3 days, respectively (28). Alongside, cinnamaldehyde and cinnamon oil is widely reported to be effective in inactivating Listeria monocytogenes, E. coli, Vibrio parahaemolyticus and many other bacterial strains in a variety of food models (19-22). Considering all of the previous findings, we can also claim CEO and CN to be a potential inactivating agent against S. typhimurium and L. monocytogenes, lending credence to their prospective application as a natural antimicrobial for meat preservation.
The findings of current study in agreement with preceeding data suggest that both CEO and CN are potential antimicrobial agents against food borne pathogens and can be implemented as a promising natural meat preservative. Considering overall cost-to-benefit ratio, extracted cinnamon essential oil can offer a rational alternative to commercial cinnamaldehyde.
Authors are thankful to the Department of Microbiology, University of Dhaka, Bangladesh, for giving logistic supports and laboratory facilities. Authors acknowledges Centre for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh; International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) and Microbial Genetics and Bioinformatics Laboratory (MGBL), University of Dhaka, Bangladesh for supporting the study by providing bacterial strains. Authors are also thankful to Dr. M.L. Bari, Principal Research Officer, CARS, University of Dhaka, for his assistance.
The study was funded by the Grants for Advanced Research in Education (GARE); Ministry of Education, Government of People’s Republic of Bangladesh.
Conflicts of Interest
Tohura Ahmed Suchi and Israt Dilruba Mishu contributed equally and would be recognized as co-first authors.
Received: 2022/08/17 | Accepted: 2022/12/23 | ePublished: 2023/03/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |