BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1558-en.html
The worldwide rise in antibiotic resistance is one of the serious problems in the treatment of wound infections (1, 2). It is noteworthy that after trauma, burns, surgery, and prolonged hospitalization, wound infection causes significant morbidity in patients (3). Staphylococcus spp., Escherichia coli (E. coli), Acinetobacter spp., Pseudomonas aeruginosa, Enterobacter aerogenes, Micrococcus spp., Bacillus spp., and Proteus mirabilis has been proved in chronic infectious wounds (4-6). Photodynamic inactivation (PDI) is a promising and suitable strategy for controlling microbial infection resistance and treating infected wounds. This new method combines light with a photosensitizer to produce a phototoxic reaction. After illumination with a light source, photosensitizers generate reactive oxygen species, O2, and free radicals, leading to the death of microorganisms.
Further, PDI is an effective tool and a new approach for treating localized wound infections, onychomycosis, and periodontitis. More precisely, it is effective in healing the wound and accelerating tissue repair via instigating fibroblast proliferation, collagen, and elastin, in addition to increasing transforming metalloproteinases and growth factor-beta (7-11). The first introduced photosensitizer for PDI was a mixture of porphyrins later known as Photofrin. Many synthetic or natural compounds have been currently presented and used as photosensitizers, and some of them have received clinical approval, including chlorine, bacteriochlorins, and phthalocyanines (12).
In Gram-positive bacteria, the photosensitizer can simply enter bacteria to mediate PDI due to a thick and porous cell wall. In contrast, in Gram-negative bacteria, there is a thinner cell wall plus an outer membrane of lipopolysaccharide. Accordingly, penetrating the photosensitizer for mediating PDI is more challenging. For this reason, using the new type of photosensitizers to induce phototoxic reactions is an innovative approach to PDI (7, 13). Using natural compounds as a photosensitizer is important in this respect. In many PDI studies, hypericin and curcumin are natural photosensitizers (14). Saffron (Crocus sativus stigmas) is a dietary spice used as an herbal medicine cultivated in Iran, India, China, and Spain. In addition, it has anti-inflammatory and anti-proliferation (of human cancerous cells) properties. Based on the evidence, saffron is exhilarant, has positive effects on the treatment of depression, and has neurological protection and cardioprotective activities.
Furthermore, it can improve bioavailability and the absorption of drugs (15-20). According to some studies, saffron is the primary natural source of carotenoids, and picrocrocin and apocarotenoids such as crocetin, crocin (α-crocin and glycoside crocin), and safranal are known as the major bioactive compounds in this spice. Carotenoid pigments are stored in flower stigmas and are responsible for the red color of saffron. Crocin is one of the derivatives of crocetin, is highly soluble in water, and represents a strong coloring capacity. Moreover, this compound is a powerful antioxidant (15, 21). The HPLC-DAD analysis of the boiling water extract of saffron confirms the presence of caffeic acid, ferulic acid, rutin, apigenin, and safranal in saffron (22). Further, aroma-active compounds have been identified by GC-MS analysis in saffron, including 2,6,6-Trimethyl-1,3- cyclohe-xadiene-1- carboxaldehyde (safranal), 2-Hydroxy-4,4,6- trimethyl-2,5- cyclohexadiene-1-one, Isophorone, 4-Ketoisophorone, and Dihydroo-xophorone (23). Crocin and crocetin demonstrate significant anti-proliferation effects on breast, lung, leukemic, and pancreatic cells (7, 17). Due to the presence of these carotenoids, saffron has visible absorption peaks at 425-452 nm, a blue light wavelength (9, 15, 24).
Considering the explanations mentioned above, the present study sought to evaluate the phototoxic reaction in Staphylococcus aureus (S. aureus), and E. coli strains after PDI using the saffron extract as a natural photosensitizer in combination with blue light.
Microbial Strains and Compounds
As a natural photosensitizer, crocus sativus stigmas (Saffron) were purchased from Kakhk, Gonabad, Razavi Khorasan, Iran. Likewise, plate count agar was provided from QUELAB. Additionally, bacterial standard strains such as S. aureus (PTCC No: 1112), E. coli (PTTC No:1330), and Candida albicans (C. albicans, PTCC No: 5027) were obtained from the Persian Type Culture Collection (PTCC) of the Iranian Research Organization for Science and Technology. Isolated strains, including S. aureus (No: 4310) and E. coli (No: 322), were prepared from Shams Laboratory in Bu in Zahra, Qazvin, Iran.
Eventually, the light source was a blue LED 9W, 230VAC/50Hz/E27 from Parsschwan Company, Tehran, Iran.
Extraction Method
Dried saffron was grounded and stored at 4-8ºC. One gram of powdered saffron was brewed in boiled distilled water for 1 h. The suspension was centrifuged at 3000 rpm at room temperature for 5 minutes. The supernatant was separated and dried, and finally, different concentrations (5-20 mg/mL) of the saffron extract were prepared and stored at -20ºC.
Determination of the Maximum Absorption Wavelength (λ max) of Brewed Saffron
The brewed saffron solution was prepared at the concentration of 0.4 mg/mL to determine the λ max. The absorption of this solution was measured in the range of 300-600 nm using a microplate ELISA reader (Bio Tek Instruments, USA) according to previous research (24).
PDI Experiments
Overnight culture of each strain was used to prepare the microbial suspension in sterile physiologic saline with the 0.5 McFarland turbidity standard. An aliquot of 100 µL of the microbial suspension was put in a 96-well microplate and incubated with 100 µL of the saffron extract's final concentration (2.5-10 mg/mL) for 15 minutes in the darkroom. After incubation, each well was exposed to 95.5 J/cm2 of blue light (9W/400-430 nm) for 5 minutes. Finally, 100 µL of microbial suspensions were spread on plate count agar in 10-5 serial dilutions. Next, colonies were counted after incubation at 37 ºC for 24 h and compared with the control plates. There were control treatments, including L-P- (cells were treated without light or photosensitizers), L+P- (cells were treated only with a light source), and L-P+ (cells were treated only with different concentrations of photosensitizers) in all experiments, which were repeated three times (8).
Statistical Analysis
The obtained data from the PDI assay were analyzed with Excel (version 2016) and Minitab (version 16) software using a sample T-test, one-way ANOVA, and then Tukey's test. P-value˂0.05 was considered statistically significant.
This study focused on investigating the potential of brewed saffron for use as a natural photosensitizer in combination with blue LED to induce the phototoxic reaction.
The absorbance spectrum of the brewed saffron showed that the maximum absorption wavelength is between 425 and 460 nm (Figure 1).
Figure 1. Absorbance spectrum of brewed saffron at 0.4 mg/mL
Based on this result and that of previous studies about the maximum absorbance of saffron (24), a blue LED was chosen as a light source in this study.
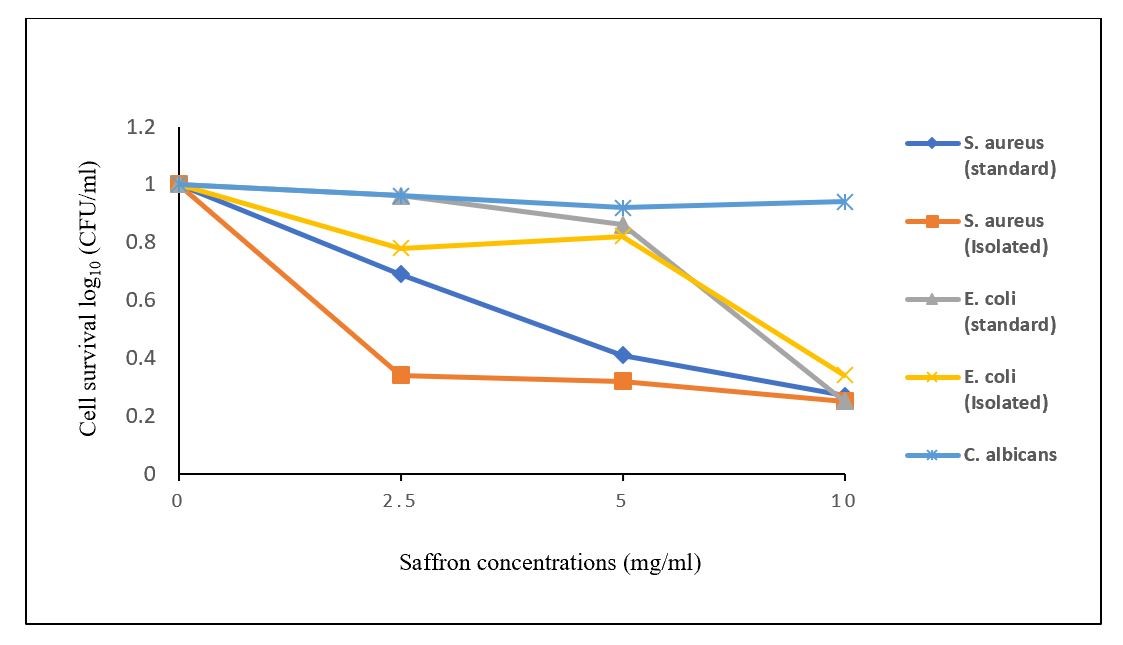
First, brewed saffron was evaluated for toxicity, and the results revealed that brewed saffron, at different concentrations (2.5-10 mg/mL), had no cytotoxic effects on the studied microbial strains after incubation for 15 minutes. Additionally, blue LED illumination for 5 minutes could not alone induce the phototoxic reaction in the intended microbial strains (Figures 3 and 4). Although different concentrations of brewed saffron induced this reaction in standard and isolated bacterial strains after 15 minutes of incubation and 5 minutes of exposure to the blue LED, these conditions could not induce the phototoxic reaction in the C. albicans strain (Figure 2).
Figure 2. Comparison of the percentage of the microbial cell survival in different bacterial and fungal strains after treatment with different concentrations of saffron in combination with blue LED
In the Gram-negative and Gram-positive strains, the highest phototoxicity was observed at 10 mg/mL of the saffron extract with blue LED illumination for 5 minutes.
After PDI, a significant difference (P<0.05) was found between L+P+ treatment and the control treatments in a standard S. aureus strain, while there was no significant difference (P>0.05) between the results of L+P+ treatments at 5 and 10 mg/mL in comparison with the 2.5 mg/mL of the saffron extract (Figure 3).
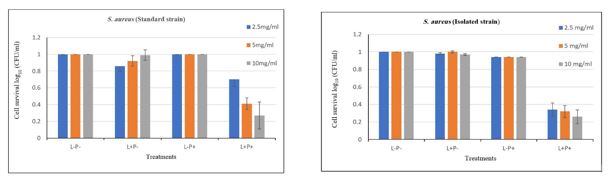
Figure 3. Comparison of the treatments (L-P-, L+P-, L-P+, & L+P+) of standard and isolated S. aureus strains after PDI with different concentrations of saffron in combination with blue LED.
Note. PDI: Photodynamic inactivation; S. aureus: Staphylococcus aureus. L-P-: Bacterial cells were treated without light and photosensitizers; L+P-: Bacterial cells were treated only with the light source; L-P+: Bacterial cells were treated only with different concentrations of photosensitizers; L+P+: Bacterial cells were treated with the light and photosensitizer. Colony count at 10-5 of serial dilution.
There was a significant difference in L+P+ treatment at the 10 mg/mL concentration with other concentrations of the saffron extract in combination with blue LED for 5 minutes in standard and isolated E. coli strains (P<0.05). The results showed that an increase in the concentration of the saffron extract at the same incubation time with the saffron extract and similar exposure time with blue LED could increase the phototoxic reaction in Gram-negative strains (Figure 4).
In the only C. albicans fungal strain, no significant difference was observed between PDI treatment and control treatments at each concentration of the saffron extract.

Figure 4. Comparison of the treatments of standard and isolated E. coli strains after PDI with different concentrations of saffron in combination with blue LED
Note. PDI: Photodynamic inactivation, E. coli: Escherichia coli. L-P-: Bacterial cells were treated without light and photosensitizers; L+P-: Bacterial cells were treated only with the light source; L-P+: Bacterial cells were treated only with different concentrations of photosensitizers; L+P+: Bacterial cells were treated with the light and photosensitizer. Colony count at 10-5 of serial dilution.
Saffron (Crocus sativus stigmas) as an herbal medicine was traditionally used in Ancient Persian and Egyptian medicine to treat measles, dysentery, and aphrodisiac, as well as inflammations, wounds, and abscesses. Some current studies have confirmed saffron's antioxidant, anticancer, antitumor, antidiabetic, and antigenotoxic properties (21, 25), which is known as a special food flavoring. In addition, it includes two important compounds, crocin and crocetin, which have high absorption at 425-452 nm, which is the wavelength peak of the blue LED. These compounds are responsible for the red color of saffron (24, 26). In this study, blue LED was used to evaluate the ability of brewed saffron to induce a phototoxic reaction in S. aureus and E. coli strains.
To our knowledge, this is the first study about PDI using brewed saffron as a natural new photosensitizer in combination with blue LED as a light source. The incubation of standard and isolated strains with different concentrations of saffron for 15 minutes and then illumination with blue LED for 5 minutes (95.5 J/cm2) effectively reduced cell survival in Gram-positive and Gram-negative strains. After PDI at different concentrations of saffron, cellular viability decreased by 0.04- 0.75 log10 (CFU/mL) in both Gram-positive and Gram-negative strains.
The anti-inflammatory and antioxidant properties of saffron may have affected wound healing (27). Several studies reported that PDI is effective in wound healing by decreasing bacterial counts in the wounds (28). The present study focused on investigating S. aureus and E. coli as two major bacteria in infected wounds (4, 28-30), and the results indicated that saffron-mediated PDI and blue LED can be effective on infected wounds with a decline in bacterial counts. Therefore, PDI and other properties of saffron can improve wound healing.
An increase in the concentration of saffron was more effective in mediating PDI, especially in E. coli strains. As mentioned earlier, crocin and crocetin are saffron's most important bioactive compounds. These compounds and their isomers belong to carotenoids with lipophilic properties, helping penetrate saffron bioactive compounds into the outer membrane of Gram-negative bacterial cells (9, 26). This feature can be a reason for the phototoxic effects of blue LED that is mediated with saffron extracts.
In their study, Garcia et al. evaluated the photoactivity of hypericin (a natural photosensitizer) against clinically isolated S. aureus and E. coli. Although hypericin significantly affected S. aureus strains, it could not induce a significant phototoxic reaction in E. coli strains (31).
Similarly, Parvathy et al. reported that the induction of the phototoxic reaction by curcumin (another natural photosensitizer) is 300 times more effective against S. aureus strains than E. coli strains (32). Flavin derivatives are natural cationic photosensitizers. In another study, Maisch et al. concluded that PDI with flavin derivatives at 50 µM combined with the light source at 450 nm resulted in a decrease of about 5 and 6.5 log10 in methicillin-resistant S. aureus and Enterohemorrhagic E. coli, respectively. In our study, mediated PDI by different concentrations of saffron exerted a similar effect on Gram-negative and Gram-positive strains. However, there was no significant difference between S. aureus and E. coli strains after PDI with saffron combined with blue LED (P>0.05). For example, the incubation of bacteria at 10 mg/mL of the saffron extract in combination with blue LED caused 0.6-0.7 log10 reductions in S. aureus and E. coli strains, while no phototoxic reaction was detected in the C. albican strain at the same condition. Moreover, our study observed no significant difference between standard and isolated strains regarding the cell survival percentage in Gram-positive and Gram-negative groups (P>0.05).
Bixa orellana is a source of natural red color that is named anatto, which is the second most important and commercially red color after saffron. Gonçalves et al. also investigated the reduction of halitosis after photodynamic therapy (PDT) when using the Bixa orellana extract with red color as a photosensitizer, and the blue LED as a light source. Halitosis is a problem that occurs because of the presence of bacteria in the coating of the tongue dorsum. They found that PDT with Bixa orellana in combination with blue LED could immediately reduce halitosis by removing bacteria (9). Additionally, in our study, PDI with different concentrations of the saffron extract with red color in combination with the blue LED induced a phototoxic reaction in bacterial strains and thus decreased cellular viability.
As previously mentioned, the global increase in antibiotic resistance is a critical problem for treating infectious diseases. The use of photodynamic inactivation is considered a promising approach for eliminating antibiotic-resistant microorganisms. This study showed that saffron has the potential as a new and natural photosensitizer because it can mediate a phototoxic reaction in bacterial strains using blue LED. In addition, it is accessible and has anti-inflammatory and antioxidant properties. The findings revealed that PDI mediated by saffron and blue LED reduces bacterial counts. Thus, it can be used as a simple technique for improving wound healing.
The author would like to use this opportunity to appreciate the Managers of Buin Zahra Ice Co. for kindly supporting us and allowing the experiments to be performed in the Company's lab. Special thanks go to Dr. Zahra Mousavian for her help in statistical analysis. The author would like to thank Mrs. Ayoubi and Shams Lab for their help in preparing the microbial strains for this project.
This research received no specific grant.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Received: 2021/12/22 | Accepted: 2022/05/17 | ePublished: 2022/09/9
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |