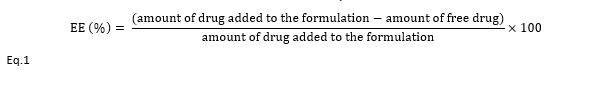BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1415-en.html

 , Ali Hossein Rezayan
, Ali Hossein Rezayan 

 2, Hale Alvandi1
2, Hale Alvandi1 

 , Mohammad Barshan Tashnizi1
, Mohammad Barshan Tashnizi1 

 , Hossein Sabahi1
, Hossein Sabahi1 


2- Division of Nanobiotechnology, Department of Life Science Engineering, Faculty of New Sciences & Technologies, University of Tehran, Tehran, Iran , ahrezayan@ut.ac.ir
Infectious diseases are recognized as one of the leading causes of death worldwide for all ages. Gastrointestinal, respiratory, genital, and nosocomial infections are the leading causes of death in developing countries (1, 2). About a century ago, the advent of antibiotics led to advances in the treatment of infectious diseases. However, the use of antibiotics has limitations, such as improper concentration of the drug at the site of infection and some side effects. Their widespread use and abuse have also led to problems such as the resistance of microorganisms to antibiotics (1, 3). Among resistant microorganisms, methicillin-resistant Staphylococcus aureus, vancomy-cin-resistant Enterococcus, vancomycin-resistant S. aureus, penicillin-resistant Streptococcus pneumo-niae, and Escherichia coli are among the common infections. Among these bacteria, the incidence and mortality of S. aureus and E. coli are significantly higher (3).
Various mechanisms have been proposed to explain bacterial resistance to antibiotics. Mechanisms of resistance of these bacteria include altered porins, increased expression of concentration-dependent pumps, the altered structure of the target site of antibiotics, and beta-lactamase production (4, 5). The antibiotic cefazolin from the cephalosporins group is active against a wide range of gram-positive bacteria and some gram-negative bacteria. This antibiotic prevents the synthesis of peptidoglycan. In recent years, antibiotic resistance has also developed in this group. In addition, the half-life of this drug is low in plasma (6, 7).
One way to combat antibiotic resistance is to use nanocarriers. By maintaining the structure of the drug, nanocarriers increase stability, increase uptake, modify the pharmacokinetics and pharmacodynamics of the drug, and increase cell penetration. The desired therapeutic effect is achieved by improving the bioavailability at low concentrations, which will reduce side effects and concentration-dependent toxicity. Also, with targeted drug delivery, the site of infection receives the maximum amount of drug, and the optimal antimicrobial effect is created. The advantages of nanocarriers include the possibility of continuous and controlled drug release, which can reduce antibiotic resistance (4, 8). One of the most critical nanocarriers in drug delivery is the noisome nanosystem; Niosomes are non-ionic surfactant vesicles composed of cholesterol and other lipids. With its two parts, hydrophilic and hydrophobic, this nanosystem can contain drug molecules with different solubilities. Niosomic surfactants are biocompatible and biodegradable (9-12). Niosomes are used as nanocarriers to deliver drugs, vaccines, and genes (13). Doxorubicin-containing niosomes provide long-term drug release and do not cause pulmonary side effects in mice. This nanocarrier doubles the antitumor activity of the drug (14). Niosome is also suitable for controlled ocular delivery of water-soluble topical antibiotics such as gentamicin (15). Niosomes loaded with antibiotics such as azithromycin, clarith-romycin, and cefixime were also synthesized using cholesterol with various surfactants (16).
Among the factors influencing the preparation of niosomes are the nature and structure of surfactants, cholesterol content, and the ratio of compounds used in their manufacture. Due to the increasing use of cefazolin in treating bacterial infectious diseases and the continuation of the authors' research on previously published drug delivery systems (17-19), this study aimed to prepare niosomal nanoparticles loaded with cefazolin with three different formula-tions and compare antibacterial activity.
Niosome Preparation
This experimental study was performed in 2018. According to previous studies, the formulation of niosome nanoparticles was prepared using three surfactants, span 60, tween 60, and cholesterol (Merck, Germany) (20-22). Table 1 shows the three formulations of niosome nanoparticles. These mater-ials were dissolved in 10 mL of chloroform and stirred in a balloon connected to a rotary evaporator (55°C and 120 rpm). After 90 minutes under vacuum, a white layer formed on the bottom of the balloon. To prepare nanoparticles loaded with cefazolin, 10 mL of phosphate-buffered saline (PBS) containing cefazolin was added after placing the balloon in a vacuum at 55°C and 55 rpm. After 40 minutes, a white layer formed on the bottom of the balloon. The nanoparticle suspension was then placed in a 150-watt sonicator for 4 minutes to reduce the size of the formed niosomes (22). Figure 1 shows a schematic represent-tation of the preparation of niosomal nanoparticles.
Table 1. Formulations for the preparation of niosome nanoparticles.
| Concentration (µmol/mL) | Ratio | Amount (g) | Material |
|---|---|---|---|
| First formulation | |||
| 9.915 | 25% | 0.043 | Span 60 |
| 9.915 | 25% | 0.064 | Tween 60 |
| 19.83 | 50% | 0.077 | Cholesterol |
| Second formulation | |||
| 11.98 | 30% | 0.0516 | Span 60 |
| 11.86 | 30% | 0.077 | Tween 60 |
| 21.76 | 40% | 0.061 | Cholesterol |
| Third formulation | |||
| 13.93 | 35% | 0.060 | Span 60 |
| 13.86 | 35% | 0.090 | Tween 60 |
| 11.89 | 30% | 0.046 | Cholesterol |

Figure 1. Schematic diagram of thin layer preparation of niosome.
Characterization of Nanoparticles
Nanoparticle Morphology
SEM image determined the morphology of niosomal particles. For this purpose, some of the samples were first placed on the lamel, and after drying, the sample was layered with gold. The niosome sample is non-conductive, and low voltage is adjusted to produce the image.
Size and Zeta Potential of Nanoparticles
A dynamic light scattering (DLS) device was used to investigate nanoparticles' size and zeta potential. In a solution, the collision of particles and small molecules with solvent molecules leads to the random motion of the molecules. The motion of small particles in a fluid is called Brownian motion. The zeta potential of the vesicles plays an important role in the function of the niosome in the body and shows its stability. The amount of zeta potential is checked with a zeta sensor. The zeta potential is the surface potential of a colloidal particle moving in an electric field (23). After the synthesis of nanoparticles, the prepared sample was passed through a 0.02 μm filter, and analysis was performed with the device (SZ-100z Dynamic Light Scattering & Zeta potential analyzer - Horiba-France).
To evaluate the stability of nanoparticles, the surface charge of the niosome after 18 days and 1 month was examined by a zeta sizer.
Drug Release and Encapsulation Efficiency
Of niosome solution containing cefazolin, 1 mL was poured into a dialysis bag and then placed in 70 mL PBS. The sample was then placed in a shaker incubator at 37°C and 80 rpm. Sampling was performed in the first 6 hours every hour and then 24 and 48 hours. Sampling was also performed on days 10 and 30. In each sample, 600 μL of PBS was removed and replaced with 600 μL of isothermal PBS. To evaluate the concentration of released cefazolin, the uptake of cefazolin at 270 nm was measured using UV-Vis spectroscopy (24).
To evaluate the encapsulation efficiency (EE), 1 mL of the nanoparticles suspension was poured into a dialysis bag and placed in 100 mL PBS. After 24 hours, the absorption of the drug was assessed by UV-Vis spectroscopy (270 nm). Then, according to formula (1), the drug loading percentage was calculated (23, 25):
Microbial Evaluation
Gram-negative E. coli (ATCC 9637) and Gram-positive Staphylococcus aureus (S. aureus, ATCC 12600) were obtained from the Faculty of New Science and Technology, University of Tehran. These bacteria were cultured on Müller-Hinton agar medium (Merck, Germany). After culturing the bacteria for 24 hours, 0.5 McFarland solution was prepared, used in experiments.
MIC Assay
Nutrient broth culture medium (Merck, Germany), 1 mg/mL solution of cefazolin, and 0.5 McFarland concentration of S. aureus and E. coli were prepared first. Concentrations of 1, 3, 4, 5, 10 μg/mL of nanoparticles for E. coli and concentration of 10, 20, 30, 40, 50, 100, 150, 200 μg/mL for S. aureus in culture medium were prepared. The bacterial solution was then added to the well. Finally, the plates were placed in a shaker incubator at 37°C and 80 rpm.
Disk Diffusion Assay
After culturing each bacterium on Müller-Hinton agar medium, three wells with a diameter of 8 mm were made in the medium. In the wells, cefazolin and nanoparticles without drugs were poured as a control. In the third well, drug-loaded niosomes were added. The culture media were incubated for 16 hours at 37°C. Then the diameter of the growth inhibition zone was measured on days 1, 2, 3, and 6 (26).
Investigation of Nanoparticle Morphology with SEM Microscope
SEM microscope images in Figure 2 show that the morphology of niosomic nanoparticles with the first, second, and third formulations was spherical, and there was no significant difference in the shape of the niosomes. The size of these nanoparticles was measured between 200 and 300 nm.
Figure 2. SEM microscope images of niosomal nanoparticles prepared with the first (A), second (B) and third (C) formulations.
Investigation of Size and Zeta Potential of Nanoparticles
Particle size is an essential characteristic of drug delivery systems affecting loading and release rates (27). The particle size and zeta potential of drug-free niosomes were 140 nm and -7.44 mV, respectively. The polydispersity index of these nanoparticles was estimated to be 0.183, which indicates that the particle size has a narrow spectrum. The zeta potential of cefazolin was also evaluated as -14 mV. Because drug delivery from nanoparticles with the third formulation was appropriate, this analysis was performed only for niosome nanoparticles prepared with this formulation. As illustrated in Figure 3, the particle size and zeta potential of niosomes containing cefazolin with the third formulation were 153 nm and -24 mV, respectively. The zeta potential of drug-containing niosomes reached -21 mV after 18 days. The size of the niosomes containing cefazolin after one month was 289 nm, and their polydispersity index was 0.264. Also, the surface charge of nanoparticles reached -53 mV after one month.
Figure 3. Results of particle size (A) and zeta potential (B) of niosome nanoparticles.
Drug Release and Encapsulation Efficiency
The encapsulation efficiency of cefazolin in niosome nanoparticles was calculated using Eq 1. The encapsulation efficiency in the niosome with the first, second, and third formulations were 33%, 19.7%, and 40.76%, respectively (Figure 4). The structure and ratio of surfactants cause differences in drug encapsulation. The release rate of the drug from the nanoparticles prepared with the first formulation in the first 6 hours was 21.7%. Also, 33% of the loaded drug was released within 48 hours. During ten days, 37% of the drug was released from the nanocarrier. Drug release reached 48% after one month. The rate of drug release from nanoparticles in this formulation was slower than in other formulations related to cholesterol levels. Cholesterol increases the strength of the membrane and reduces the release of cefazolin through diffusion. In the second formulation, 35% of the cefazolin was released in the first 6 hours. Also, within 48 hours, the release reached 59.9%. The release of cefazolin in this formulation after 10 and 30 days was 80% and 81.5%, respectively. The release rate of cefazolin from nanoparticles prepared with the third formulation was higher due to the lower cholesterol ratio than the other two formulations. In the first 6 hours, 42%, and after 48 hours, 52% of the drug was released from the niosome nanoparticles. This release reached 60% and 63.1% after 10 and 30 days.

Figure 4. Cefazolin released from niosome nanoparticles after 48 hours (A) and 30 days (B).
Microbial Evaluation:
MIC Assay
The concentration of 4 μg/mL was determined as the minimum growth inhibitory concentration of E. coli. A concentration of 150 μg/mL was also determined as the MIC for S. aureus.
Disk Diffusion Assay
The drug-free niosomes did not inhibit growth in any of the bacterial strains; This result indicates that the niosomes do not have antibacterial properties. The antibacterial activity of cefazolin on the first day was significantly higher than the niosomes loaded with the drug (P<0.05). The diameter of the growth inhibition zone of this drug was measured to be 3 cm, which is related to the higher concentration of the drug in the environment. In the nanoparticles with the first formulation, the growth inhibition zone of E. coli decreased rapidly during three days, and bacterial colonies were observed on the sixth day (Figure 5). The antibacterial activity of nanoparticles with the first formulation in the first 24 h was significantly higher than other days (P<0.05). Over time, the drug is released into the culture medium, which reduces the concentration of the drug in the inhibition areas. Then, as the release of the drug from the nanocarrier decreases, the growth of bacteria prevails, and the diameter of the inhibition zone becomes small. Also, a small inhibition zone was observed on the first day in the S. aureus bacterial plate, which disappeared in the following days.
Niosome nanoparticles with the second formulation created a growth inhibition zone in the E. coli with a diameter of 1.85 cm. The load and drug released from the niosome with the second formulation were less than the other formulations. The antibacterial activity of niosomes with the second formulation on the first day was significantly higher than other days (P<0.05). However, the antibacterial activity in the first 24 hours was not significant compared to nanoparticles pre-pared with the first formulation. Niosome with a second formulation did not create a measurable inhibition zone for S. aureus.
The growth of E. coli treated with the third niosome was also reduced. On the first day, the growth inhibition zone of this nanocarrier was 2.2 cm, which was not significant compared to the first formulation. However, the inhibition zone with the third formulation remained almost the same until the sixth day, and its antibacterial activity was significantly higher than other niosomal nanoparticles (P<0.05). The drug loading and release from this nanocarrier was more than the other two formulations; As a result, the nanocarrier with the third formula had an excellent antimicrobial effect against E. coli in 6 days. The growth area in the S. aureus was more significant than the diameter of the first formulation (P<0.05). However, this area disappeared in the following days.
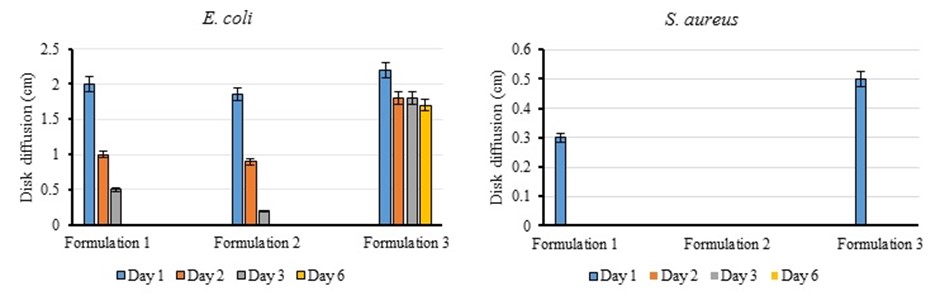
Figure 5. Comparison diagram of disk diffusion test results for 3 niosomes formulations on E. coli and S. aureus.
Among the advantages of nano antibiotics over conventional antibiotics are increased solubility and suitable destruction in the body. Therefore, the desired therapeutic effect can be achieved by improving bioavailability at lower doses (4). There are many hypotheses about the antimicrobial mechan-isms of nano antibiotics; Increasing the surface-to-volume ratio seems to increase the level of contact with the bacteria. These antibiotics also produce large amounts of reactive oxygen species, and their unique formulation reduces drug release from bacteria (28). Much attention has been paid to vesicular drug delivery systems, such as liposomes and niosomes. Studies show that niosomes are effective, targeted, and long-lasting drug delivery systems than liposomes (29). Due to their non-ionic nature, niosomes are more compatible and less toxic than other drug delivery systems (30). Studies show that the type and concentration of surfactants used to synthesize niosomes play an essential role in encapsulating and releasing drugs from nanoparticles. As the surfactant chain length increases, the encapsulation rate increases (29). In this study, niosomic nanoparticles containing the antibiotic cefazolin were synthesized using three formulations containing span 60, tween 60, and cholesterol in different ratios, and the properties of nanoparticles and their antibacterial activity were investigated.
The morphology of the synthesized nanoparticles was spherical with all three formulations. The size of these nanoparticles was slightly different in SEM and DLS. This difference may be due to differences in how nanoparticles are prepared and sampled. Dharashivkar et al. also observed that the niosomal nanoparticles prepared with span 60 and tween have a spherical structure (31). Yaghoobian et al. (2020) observed that the size of niosome nanoparticles synthesized with different surfactant varies between 119 and 236 nm. The size of nanoparticles synthesized with spin 60 and tween 60 was 212 nm (32). The polydispersity index of nanoparticles is between 0 and 1. The closer this number is to 0.1, the particle size is more uniform.
The zeta potential of all nanoparticles in this study was negative. With the loading of cefazolin in the niosome, the zeta potential changed from -7.4 to -24 mV. This change is related to the zeta potential of the cefazolin. Cefazolin is first placed in the hydrophilic part of the niosome, and over time, due to the dynamics of the membrane, it is also placed in the membrane, causing the zeta potential to become more negative. Zeta potential greater than ±30 mV indicates good stability, the zeta potential of ±20 mV indicates low stability, and nanocarrier instability is in the range of ±5 mV (22, 33). The zeta potential of the synthesized nanoparticles shows their average stability. Also, loading the drug in the nanoparticles increases the particle size from 140 to 153 nm. In another study, it was observed that the zeta potential of niosomal nanoparticles reached -25 mV after loading of carvedilol. Also, the release of carvedilol in this system increases the particle size to 167 nm (22). The nanoparticle size increased to 289 nm after one month, but the polydispersity index shows particle size uniformity. In 2017, Taymouri et al. observed that the size of niosome nanoparticles increased from 167 to 389 nm after one month. The polydispersity index increased from 0.6 to 0.9, which means an increase in size and high dispersion of particle size. The zeta potential of these nanoparticles also changed from -25 to -17 mV (22).
The amount of cefazolin loaded in the niosome in formulation 3 was higher than the other two formulas (40.76%). Uchegbu et al. found that the loading efficiency of doxorubicin in niosome nanoparticles consisting of span 60, cholesterol, and choleth24 at 45:45:10 was 35% (14). The size and physicochemical properties of the drug molecule are critical factors in drugs released from nanoparticles. The niosome synthesis method also affects its encapsulation efficiency. Drug release from the niosome in the first formulation was slow. These results are consistent with other studies (13, 34, 35). By lowering cholesterol from the first to the third formulation, the release rate increases. Cholesterol in the structure of the niosomal membrane increases the strength and decreases the rate of drug release. In this experiment, the niosomes with the third formula, which have the highest drug load and contain less cholesterol, release more drugs than the niosomes of the other two formulas throughout the release period.
Niosomes containing cefazolin inhibit the growth of E. coli more than S. aureus significantly (P<0.05). This difference is related to the difference in the structure of the bacterial cell wall. Through their defense mechanisms, such as beta-lactamase production and increased concentration-dependent pumps, bacteria destroy the antibiotic's structure and reduce its concentration in the environment. Bacteria that have escaped the drug then multiply rapidly. One of the essential effects of antibiotic loading on nanoparticles is the stability and maintenance of drug structure (36, 37). Due to the lower loading and release rate, the nanoparticles synthesized with the second formul-ation create a smaller inhibition area. Low drug release in this formulation also allows bacteria to multiply; For this reason, the diameter of the inhibition area decreases rapidly. Niosomal nano-particles synthesized with the third formulation have a more significant inhibitory effect against both bacterial strains due to the higher loading of cefazolin. The antibacterial effect can be observed over time with cefazolin's continuous and controlled release from these nanoparticles. Another mechanism that increases the antibacterial activity of nanoparticles loaded with antibiotics is the penetration of nanoparticles into the bacterial cell. Niosomes interact with bacterial cells through fusion and adsorption. Also, reducing the size of the niosomes results in better interaction with the bacterial membrane and reduces the MIC values (35, 38). The lipid layer of the niosomes fuses with the bacterium's outer membrane, and the drug accumulates in the bacterial cell. Accumulation of nanoparticles and drugs in bacterial cells also causes irregularity in the cytoplasmic membrane and its rupture (39). Also, with the optimal entry of this antibiotic into bacterial cells and dysfunction of proteins, peptidoglycan wall synthesis is effectively inhibited (40). Table 2 com-pares the anti-bacterial effect of nanocarriers cont-aining antibiotics.
Table 2. Comparison of antibacterial effect of antibiotic nanocarriers on the growth of gram-positive and gram-negative bacteria.
| Antibiotic | Nanocarrier | Results | Ref. |
| Norfloxacin | Solid lipid nanoparticles | Nanocarriers containing antibiotics significantly reduce the growth of E. coli compared to free drug and this effect is stable after 9 months (MIC value = 0.2 μg/mL). | (41) |
| Florfenicol | Solid lipid nanoparticles | The MIC of nanocarriers containing antibiotics for E. coli and S. aureus are 3 and 6 μg/mL, respectively. This nanocarriers significantly reduces the mortality of mice with E. coli compared to the free drug. | (37) |
| Cefazolin | Niosome | Niosomes containing cefazolin at concentrations of 128 and 256 μg/mL removed S. aureus 3 and 5-day biofilms. | (42) |
| Cefazolin | Single-layer liposomes | The use of this nanocarrier reduced the population of S. aureus by 7.9 times. | (43) |
| Cefazolin | Niosome | The MIC of nanocarrier containing antibiotics for E. coli and S. aureus were 4 and 150 μg/mL, respectively. | Present study |
Increasing the use of new drug carriers is a crucial way to increase antibiotic efficacy, reduce dose, and thus reduce the risk of developing antibiotic resistance. In this study, niosomal nanoparticles containing cefazolin were prepared by the thin layer hydration method. Niosome nanoparticles were fabri-cated with three different formulations and ratios of cholesterol, span 60 and tween 60. Antibiotic encapsulation efficiencies in nanoparticles with first, second, and third formulations were 33%, 19.7%, and 40.76%, respectively. The morphology of the synth-esized spherical nanoparticles and the size and zeta potential of the particles were 154 nm and -24 mV, respectively. The release of cefazolin from the first, second, and third niosomal nanocarrier during 30 days was 48%, 81.5%, and 63%. The niosomal nanocarrier prepared with the third formulation showed a good antibacterial effect against Escherichia coli for 6 days, and the diameter of its growth inhibition zone remains almost constant. The minimum inhibitory concen-trations for Escherichia coli (E. coli, ATCC 9637) and Staphylococcus aureus (S. aureus, ATCC 12600) were measured at 4 and 150 μg/mL, respectively. The niosomal nanocarrier entered the bacterial cell by attaching to the outer membrane of the bacteria and preventing the growth of bacteria by the continuous and controlled release of antibiotics.
The authors would like to acknowledge the Iran National Science Foundation (INSF) (Project 96002094) for the financial support of this work.
Conflicts of Interest
There is no conflict of interest between the authors in the study.
Received: 2021/08/6 | Accepted: 2021/11/6 | ePublished: 2021/12/8
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |