BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1205-en.html
2- Department of Microbiology, Faculty of Basic Sciences, Shahrekord Branch, Islamic Azad University Shahrekord, Iran , bamzadehz@yahoo.com
3- Department of Biology, Faculty of Science, Farhangian University, Tehran, Iran.
4- Cellular and Developmental Research Center, Faculty of Basic Sciences, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
.
Today, cancer is one of the most important health challenges all over the world and 25% of death cases in the United States are due to cancer. Half of all the cancer cases occur in Asian countries which make 60% of the world population. It has been estimated that the cancer cases will increase from 6.1 million in 2008 to 10.6 million in 2030, which is due to the significant growth of the world's oldest population, and changes in the lifestyle and economic changes in the society (1). In the developed countries, the prostate cancer is the second most common cancer (after skin cancer) and the second deadliest cancer (after lung cancer) in men. So that, one in six men develops prostate cancer. The epidemiological studies have also shown that hereditary factors play a role in the incidence of 10% of these cases. The highest and the lowest prevalence of prostate cancer were found in Africa and Asia, respectively (2). On the other hand, the first gene locus known to be associated with the prostate cancer was the Hereditary Prostate Cancer locus-1 (HPCL). Several other candidate genes have been identified since this discovery, although most of them are less important due to their low frequency. Most of the prostate tumors are adenocarcinomas. The hereditary history of prostate cancer is an important factor in the development of this cancer. The hereditary factors are involved in a small percentage of prostate cancers and are usually associated with the early onset. In men, increased androgen levels are associated with an increased risk of prostate cancer. The androgen receptor gene plays an important role in the development and progression of prostate cancer. Also, AR, HSD3B, 2HSD3B1, SRD5A2, and CYP17 genes have a special role in the cell proliferation in androgen metabolism in prostate.
Some polymorphisms in these genes are associated with an increased risk of prostate cancer. Mutations in androgen receptor are found in almost all cases of prostate cancer, and treatment strategies for this cancer emphasize on the reducing or eliminating testosterone binding to the androgen receptor. The epigenetic changes, especially DNA hypermethylation in the promoter regions, play an important role in reducing the expression of genes important for the care and prevention of prostate cancer. A number of molecular and genetic changes have been observed in prostate cancer. These factors play a role in the onset and progression of prostate cancer. Metastasis-inhibiting genes are also known in prostate cancer. The hypermethylation of promoter regions of some genes involved in apoptosis is also known in cancer.
Prostate cancer, in fact, is the presence of cancer cells in this organ, for the increased androgen level of the adrenal glands, which cause obstruction in the urinary tract (3, 4). The main alarming signs of the prostate cancer include: frequent or difficult urination, poor urine flow, inability to urinate, urinary incontinence, intermittent and poor urine flow, blood in the urine, painful ejaculation, persistent low back pain and sexual impotence (5). Regardless of the gender or racial origin, the strongest known risk factor in this case is a family history of the disease. Prostate cancer is the most common cancer diagnosed in American men. It is more likely for a man who does not smoke, to develop about 17% prostate cancer more than other cancers (6).
One of the diagnostic and screening methods for the prostate cancer is the prostate gland finger examination through the rectum.
Rapid diagnosis of the prostate cancer is mediated by measuring the prostate-specific antibody (PSA) using screening tests. The level of this antibody is higher in the patients with prostate cancer. In some cases, an infection or benign enlargement of the prostate gland can cause an increase in the PSA in the blood. Therefore, combining the anal examination with PSA level in the blood is a more accurate way to diagnose prostate cancer. In the next stages, other examinations such as MRI, ultrasound, computed tomography and gland sampling are performed (7).
Conventional treatment options for the prostate cancer include surgery (complete removal of the prostate gland or prostatectomy) (8), cold surgery or cold therapy (9), chemotherapy, radiotherapy, and gene therapy (10). There are various mechanisms by which these treatments are able to control various tumor cells, including induction of apoptosis, increased mitochondrial membrane permeability, and blocking transduction signals (processes that occur in the metabolic pathway) through inhibition of the key enzymes, cytomorphological changes due to impaired cell differentiation, and tumor-induced angiogenesis. Antitumor compounds produced by Streptomyces occasionally act by disrupting double-stranded DNA, leading to detrimental effects on the rapid cell proliferation by inhibiting DNA-dependent RNA polymerase activity (11).
Finally, despite the fact that there are different ways to treat cancer, cancer is still a common and public concern. Chemotherapy is one of the most effective ways to control cancer, but it requires a constant supply of the new antitumor compounds. Cancer treatment is still a major issue for the physicians and the pharmaceutical industry. More than 60% of the chemotherapeutic agents used today to treat cancer are compounds that have been identified from the soil microorganisms or compounds synthesized or derived from them with the modified properties (12). Because soil is the home to a variety of living organisms, including bacteria, fungi, protozoa, nematodes, insects, and many other living organisms. The bacterial population can reach 108 to 109 cells per gram of dry soil weight at soil surfaces. Soil bacteria are very diverse and can be aerobic, anaerobic or optional anaerobic. In addition, they can be heterotrophic or autotrophic in terms of nutrition (2).
Studies have shown that the soil and rhizosphere contain thousands of unique bacterial species per gram of soil. However, today only a very small part of these bacteria that are capable of producing secondary biological metabolites have been studied.
Studies have shown that strains of bacteria are able to produce a wide range of secondary metabolites encoded by gene clusters that are not expressed in vitro. Secondary metabolites are not directly involved in the growth, development, or proliferation of the bacteria, but these metabolites may play ecological roles in the interaction of these bacteria with other organisms (13). In other words, many microbial metabolites are essential drugs in the treatment of cancer. Their therapeutic application began from 1940 with the discovery of actinomycin, and since then many new compounds with anti-cancer properties have been identified and isolated from natural sources (12).
Finally, due to the increasing cancer prevalence, which is one of the most important and common causes of death in human, there is a need to discover new compounds and create independency in the production of anti-cancer metabolites and being independent from foreign countries. On the other hand, few studies have been done on the effect of microbial production metabolites on cancer cells. Also, the society needs to identify new biologically active compounds produced by endemic bacteria. In this study, the anti-cancer effects of metabolites extracted from Bacillus licheniformis isolated from the soil of Chaharmahal and Bakhtiari province was investigated against prostate cancer cell line (PC-3).
Isolation and Identification of Gram-positive Bacteria from Soil Samples
During this study, 70 soil samples were collected from different regions of Chaharmahal and Bakhtiari province in the spring of 2019 from the depth of 10 to 15 cm next to the plant roots and transferred to the laboratory to isolate and identify the gram-positive bacteria (14). To identify their phenotype, following culture and purification of bacteria, the macroscopic and microscopic characteristics of each isolate were examined (15, 16). After initial identification, the best isolate in terms of antimicrobial compounds production with a wider range of effects was identified using molecular identification method.
Molecular Identification of Gram-positive Bacteria Producing Antimicrobial Compounds
The 16S rRNA gene sequence was used for the genotypic identification. First, DNA extraction was performed using DNA extraction kit (CinnaGen, Iran). Then, to ensure the purity of DNA, the light absorption of the sample was measured at 260/280 nm wavelength. If the DNA sample is pure, the ratio should be above 1.8, otherwise, the DNA is considered impure. Forward and reverse primers for 16S rRNA gene were synthesized by CinnaGen Company (Table 1).
| Primer | Sequence (5'→3') |
| 1000F16 | CAACGAGCGCAACCC |
| 1429R | GGTTACCTTGTTACGACT |
To prepare a master mix with a volume of 25 µL, sterile distilled water (18 µL) was mixed with 10X PCR buffer with (2.5 µL), 0.75 µL MgCl2, 0.5 µL dNTP, 1 µL forward and reverse primers (10 pmol/µL), 0.25 μL polymerase enzyme and 1 μL of template DNA. Finally, the PCR program was performed by thermal cycling apparatus (Eppendorf, Germany). To start the polymerization process, the thermal cycling machine was set at 95°C for 180 sec, followed by 35 cycles of 95°C for 60 sec, 56°C for 45 sec and 72°C for 60 sec. Final elongation was performed at 72°C for 300 sec (17). To confirm the amplification of 16S rRNA gene, the PCR product was electrophoresed on 1% agarose gel. The PCR product was sent to Macrogene Co., Korea for sequencing. The obtained sequence was evaluated using BLAST software of NCBI (17).
Bacillus licheniformis Culture
In order to isolate the products, Bacillus licheniformis was cultured in TSB (Trypticase Soy Broth) medium for 48 hr, and then centrifuged at 12000 g for 10 min. The cells were separated from supernatant. The supern-atant was used to evaluate the cytotoxic activity. For this purpose, the supernatant was lyophilized for 7 hr and then dried. The samples were stored at -20°C until use (14).
Cytotoxic Effects of Bacillus licheniformis Supernatant on PC-3 Cancer Cell
The PC-3 prostate cancer cell line that is used for the prostate cancer research and drug production was obtained from the Pasteur Institute of Iran. The PC-3 cell line was derived in 1979 from grade 4 bone metastasis of prostate cancer from a 62-year-old Caucasian man. After transferring the flask to the lab, the cell media was removed and fresh medium (RPMI + FBS10% + Pen / Strep1%) (Sigma Company) was added and incubated at 37°C, 5% CO2 and 95% O2 atmosphere.
To investigate the effect of Bacillus licheniformis supernatant on the prostate cancer cells apoptosis, the cells were exposed to different concentrations. The experimental groups included: Group I: Cells were exposed to 5 mg/ml concentration of Bacillus licheniformis supernatant. Group II: Cells were exposed to 10 mg/ml concentration of Bacillus licheniformis supernatant. Group III: Cells were exposed to 15 mg/ml concentration of Bacillus licheniformis supernatant. Group IV: Cells were exposed to 20 mg/ml concentration of Bacillus licheniformis supernatant. Control group: Cells were exposed to RPMI1640 culture medium.
The MTS kit (Promega) is a quantitative and colorimetric assay. The MTS is reduced by the mitochondrial enzyme succinate dehydrogenase in living cells. Briefly, the cells were cultured into 96-well plate (5×103 cell/well). After 24 hr and cell confluency, the supernatant was replaced with new media containing different concentrations of metabolite (5, 10, 15 and 20 mg/mL for groups I, II, III and IV, respectively) and the cells were incubated for 24, 48 and 72 hr. Untreated cells were considered as control. After the incubation times, 20 μL of MTS solution was added to each well and incubated for 4 hr. The intensity of the color was determined by ELISA reader at 492 nm wavelength. All the experiments were repeated three times and the data were expressed as mean ± SD (18).
Apoptosis Assay
The apoptosis induction was assessed using FITC Annexin V Apoptosis Detection Kit with PI, product number 556547 (BD Pharmingen, USA). The concentrations of 5, 10, 15 and 20 mg/ml of the metabolite at intervals of 24, 48 and 72 hr were used to evaluate the apoptosis. The cells were trypsinized, harvested and centrifuged after washing with PBS (Gibco, USA). The Annexin-V dye bound to FITC and Propidium iodide (PI) were added (5 μL) and incubated for 15 min at room temperature in dark and evaluated by flow cytometry (FACSCalibur, USA) (18).
Statistical Analysis
Data statistical analysis was performed by SPSS software using t-test and ANOVA parametric test. To determine the significant difference between the treated groups and the control group, the P<0.05 was calculated as a significant difference and the results were shown as the mean ± SD.
Cytotoxic Results of Bacillus licheniformis Supernatant on PC-3 Cell Line
The microscopic observations showed the morphological changes and a decrease in the cell number in the treated groups in a concentration- and time-dependent manner (Figures 2 and 3).
Sequence ID: dbj|AB275356.1| Length: 1501
| Score | Expect | Identities | Gaps | Strand |
| 555 bits(300) | 2e-154 | 348/371(94%) | 4/371(1%) | Plus/Plus |
Query 16 TTGCGGC-CTCTAAGGTGACTGCCGGTGA-TAACCGGAGGAAgggggggATGACGTCAAA 73
||| ||| ||||||||||||||||||||| ||||||||||||| |||||||||||||||
Sbjct 1126 TTG-GGCACTCTAAGGTGACTGCCGGTGACAAACCGGAGGAAGGTGGGGATGACGTCAAA 1184
Query 74 TCATCCTGCCCCTTATGACCTGGGCTACCCACGTGCTACAATGGGCATAACAAAGGGCAA 133
||||| |||||||||||||||||||||| |||||||||||||||||| |||||||||||
Sbjct 1185 TCATCATGCCCCTTATGACCTGGGCTACACACGTGCTACAATGGGCAGAACAAAGGGCAG 1244
Query 134 CGAACCCCCGAGGCTAAGCCAATCCCACAAATCTGTTCTCAATTCGGATCGCAATCTGCA 193
|||| || ||||||||||||||||||||||||||||||||| ||||||||||| ||||||
Sbjct 1245 CGAAGCCGCGAGGCTAAGCCAATCCCACAAATCTGTTCTCAGTTCGGATCGCAGTCTGCA 1304
Query 194 ACTCCACTGCCTGAAGCTGGAATCCCTAGTAATCCCGGATCAACATGCCGCGGTGAATAC 253
|||| ||||| ||||||||||||| ||||||||| ||||||| |||||||||||||||||
Sbjct 1305 ACTCGACTGCGTGAAGCTGGAATCGCTAGTAATCGCGGATCAGCATGCCGCGGTGAATAC 1364
Query 254 CTTCCCGGGCCTTGTACACACCGCCCGTCACACCACGAGAGTTTGTAACACCCGAAGTCG 313
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sbjct 1365 GTTCCCGGGCCTTGTACACACCGCCCGTCACACCACGAGAGTTTGTAACACCCGAAGTCG 1424
Query 314 GTGAGGTAACCTTTTGGAACCAGCCGCCCAAGGTGGGACAGATGATTGGGGTGAAGTCGT 373
|||||||||||||||||| ||||||||| |||||||||||||||||||||||||||||||
Sbjct 1425 GTGAGGTAACCTTTTGGAGCCAGCCGCCGAAGGTGGGACAGATGATTGGGGTGAAGTCGT 1484
Query 374 A-CATGTAACC 383
| || ||||||
Sbjct 1485 AACAGGTAACC 1495
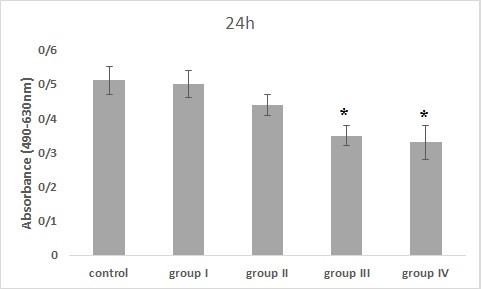
Figure 4. The effect of Bacillus licheniformis supernatant on PC-3 cell viability for 24 hr of incubation (*: P<0.05, significant difference compared to the control group).
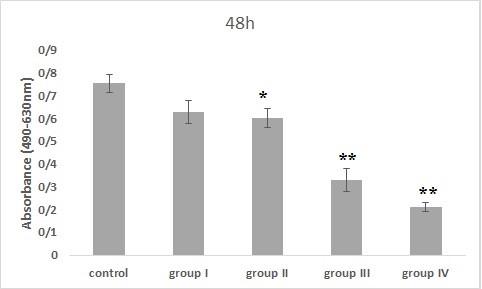
Figure 5. The effect of Bacillus licheniformis supernatant on PC-3 cell viability for 48 hr of incubation (*: P<0.01 and **: P<0.001, significant and highly significant differences compared to the control group).
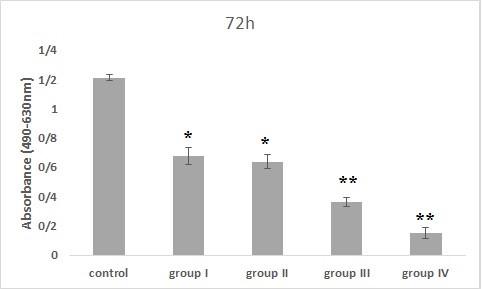
Figure 6. The effect of Bacillus licheniformis supernatant on PC-3 cell viability for 72 hr of incubation (*: P<0.01 and **: P<0.001, significant and highly significant differences compared to the control group).

Figure 7. The effect of Bacillus licheniformis supernatant on PC-3 cells apoptosis. The PC-3 cells were incubated with 5-20 mg/ml of supernatant for 24 hr.

Figure 8. Percentage of viable cells, early apoptosis and late apoptosis in PC-3 cells treated with different concentrations of Bacillus licheniformis supernatant for 24 hr. *: P-value<0.05; **: P-value<0.01 vs. control group.

Figure 9. The effect of Bacillus licheniformis supernatant on PC-3 cells apoptosis. The PC-3 cells were incubated with 5-20 mg/mL of supernatant for 48 hr.

Figure 10. Percentage of viable cells, early apoptosis and late apoptosis in PC-3 cells treated with different concentrations of Bacillus licheniformis supernatant for 48 hr. *: P-value<0.01; **: P-value<0.001 vs. control group.
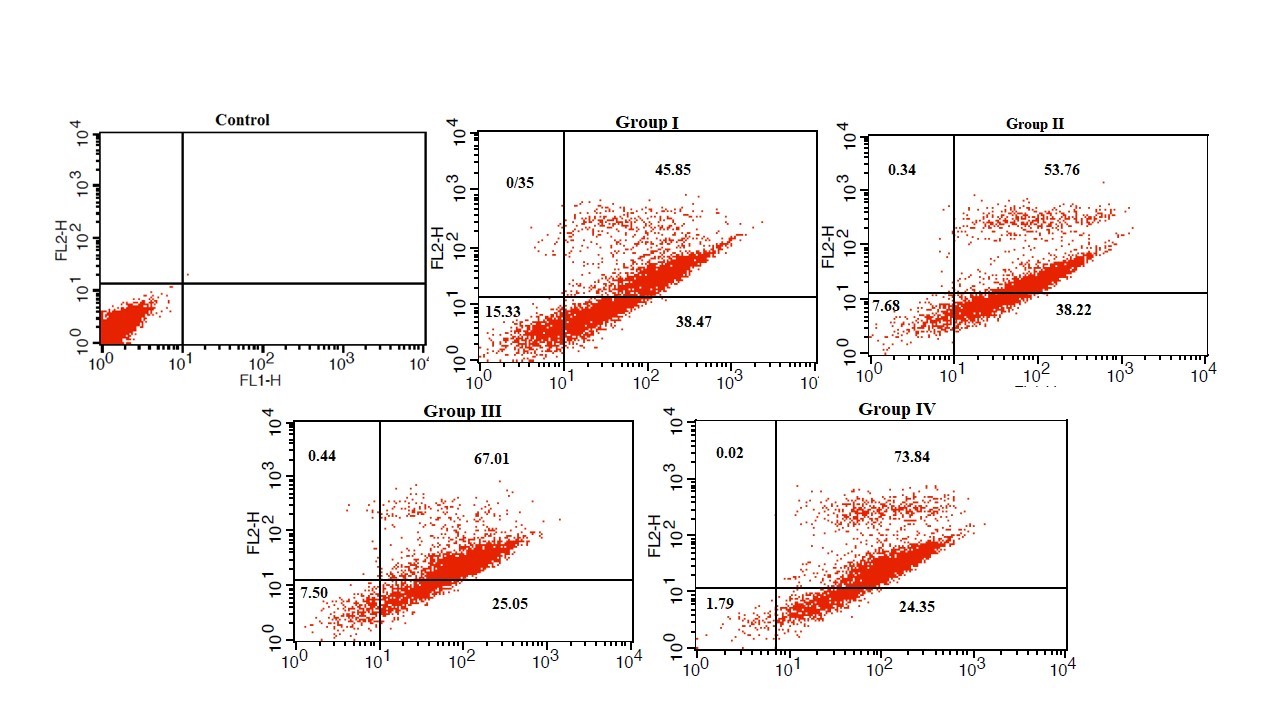
Figure 11. The effect of Bacillus licheniformis supernatant on PC-3 cells apoptosis. The PC-3 cells were incubated with 5-20 mg/mL of supernatant for 72 hr.
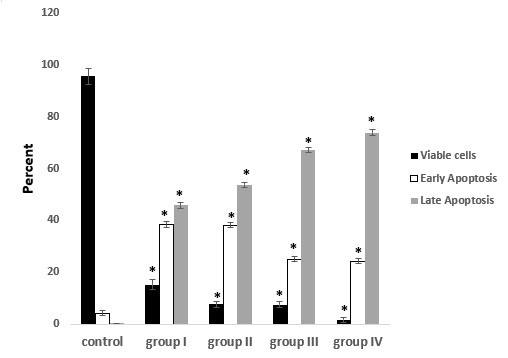
Figure 12. Percentage of viable cells, early apoptosis and late apoptosis in PC-3 cells treated with different concentrations of Bacillus licheniformis supernatant for 72 hr. *: P-value<0.001 vs. control group.
Due to the spread of cancer as one of the most important and common causes of human mortality, resistance to chemotherapy drugs and several disadvantages and sometimes oncogenic harms of these drugs, the necessity to discover new compounds and create independency from foreign countries in the field of antibiotics production and anti-cancer drugs is felt. On the other hand, due to the fact that rare studies have been conducted in the field of antitumor compounds production from endemic bacteria of Iran, there is a need to identify new endemic strains producing new compounds. Also, the ability to produce antimicrobial substances is not only a defense tool, but also from a pharmacological point of view and due to the side effects of antibiotics and resistance of pathogenic microorganisms against antibiotics, they can be used effectively in the treatment of diseases. Therefore, this study investigated the anti-cancer effects of Bacillus licheniformis products against prostate cancer cell line (PC-3) by cytotoxicity assay. In this study, Bacillus licheniformis was used as the best isolate in terms of antimicrobial compounds production with a wide range of effects to study anti-cancer activity and the effect of its compounds was investigated on PC-3 cell line viability.
The PC-3 cell line was treated with Bacillus licheniformis supernatants in 4 groups. The results of MTS assay in 24 hr incubation with the treatment showed that cell viability was dose-dependent, so that, with increasing the concentration, the cell viability decreased compared to the control group. This decrement was significant in groups III and IV compared to the control group (P<0.05). Also, in the 48 hr treatment, all groups except the first group showed significant differences from the control group (P<0.01) and finally the cells treated for 72 hr, showed a dose-dependent decrease in all groups compared to the control group. It was significant in all groups compared to the control group (P<0.001).
Phonnok et al. in 2010 investigated microbial metabolites and their role in the apoptosis induction in cancer cells. They evaluated the effect of 394 microbial extracts on the proliferative activity of 4 categories of cancer cells using MTT assay. Using these extracts, morphological changes such as cell shrinkage, loss of surface contact and loss of cell swelling were observed in all treated cancer cells (15), which can confirm the results of the current study. The cell viabilities showed a concentration-dependent decrease compared to the control group when exposed to different concentrations of supernatant for 24, 48 and 72 hr.
In 2010, Tuo et al. tested the anti-proliferative effect of 7 wild Lactobacillus strains isolated from food fermentation on HT-29 cell line isolated from laboratory models. The results showed that only Lactobacillus coryniformis strain had anti-proliferative activity on HT-29 cells (19). In the present study, different concentrations of Bacillus licheniformis supernatant were able to inhibit the PC-3 cell line growth.
Moosavi et al. in 2011 investigated the effect of the secondary metabolites form Streptomyces sp. ABRIINW 111 on K562 cell apoptosis. In their study, K562 cell was treated with different concentrations of ether-soluble metabolites for 12 to 72 hr. Trypan blue dye and DNA fragmentation test were used to investigate the growth inhibition and apoptosis occurrence, respectively. The data showed that ether-soluble metabolites inhibited the growth of K562 cell line in a concentration- and time-dependent manner (20). The current study has presented similar results to Mousavi's study in terms of examining the effect of produced compounds on cancer cell line and apoptosis. In addition, Annexin kit and flow cytometry method were used in the present study.
In a 2013 study, Parsaseresht et al. examined the effect of Lactobacillus rhamnosus GG secondary metabolites on CacoII cancer cell (colorectal cancer cell line) and showed that, Lactobacillus rhamnosus GG metabolites inhibited CacoII cancer cell growth at concentrations of 100, 200 and 300 mg/mL by 60.8%, 72.5% and 82.1%, respectively (16). These findings are in line with the findings of the present study on the anti-cancer effects of microbial compounds. In 2013, Mahmoudi Aslzadeh et al. examined the probiotic effect of Bifidobacterium bifidium on CacoII cancer cells. To this purpose, 100, 200 and 300 μL of the supernatants were applied to the CacoII cancer cells cultured in 96-well microplates. The results showed inhibitory percentage of Bifidobacterium supernatant on cancer cells at 55% to 82%, which were related to the concentration of 100 μL in 24 hr and 300 μL in 72 hr, respectively (21). In this study, selective bacterial supernatant was used and similar results were obtained.
In 2014, Vijaya Kumar et al. examined the effect of metabolites of Bacillus cereus and Bacillus popilliae isolated from the infected specimens in Shimoga, Karnataka, India, to investigate the anti-cancer and cytotoxic properties of various components. The extracts obtained from the metabolites of these two bacteria were used on normal human liver cells and 2 types of cancer cells. The results indicated that both samples had significant activity. However, the sample taken from Bacillus cereus showed a high cytotoxic effect, but the metabolites of Bacillus popilliae had toxic effects on normal and cancer cells (22). These results are similar to the present research, except that in the present study no toxic effects were observed on normal cells.
In 2015, Vazquez-Rivera et al. in Mexico investigated the cytotoxic effects of the dipeptide cycle of Pseudomonas aeruginosa PAO1 on apoptosis in human cancer cell lines. These compounds showed 50% inhibitory effect (23), which confirms the results obtained in the current study.
Pasiar et al. in 2016 studied the selective growth inhibition of the breast adenocarcinoma cell line using metabolites produced by the endemic strain Pseudomonas UW4 sp. in the laboratory. In this study, the antimicrobial activity of the metabolites of the endemic strain Pseudomonas UW4 sp. was investigated against some pathogenic bacteria. To evaluate the anti-cancer activity, SKBR3 and HU-02 cells were treated with different concentrations of 5, 10, 15 and 20 mg/mL for 24, 48 and 72 hr. The obtained results showed that Pseudomonas UW4 sp. was able to produce antimicrobial metabolites against Staphylococcus aureus. Metabolite treatment showed that by increasing the concentration in a dose- and time-dependent manner, the viability of the cells decreased. The maximum effect was related to 20 mg/ml concentration at 72 hr treatment. While metabolites at different concentrations did not have a significant effect on normal fibroblasts (18), these results confirmed the present study results.
Zhang et al. in 2017 examined the antitumor property of Rhodococcus sp. Lut0910 isolated from contaminated soil. The effects of those metabolites were investigated on two categories of liver cancer cells (HepG2) and uterine cancer cells (Hela). Treatment of these cancer cells with the bacterium Rhodococcus Lut0910 extract delayed cell proliferation in a dose-dependent pattern. Also, oral administration of the mentioned bacterial extract to the mice with specific tumors resulted in inhibition of the tumor growth in comparison with the control group (1). This study outcome in the field of cytotoxic effects of microbial production compounds was in line with the present study.
Dehnad et al. in 2017 investigated antimicrobial, chemical and histopathological properties of the secondary metabolites of Streptomyces levis isolated from the soils in the Northwest of Iran in in-vitro and in-vivo conditions. Their data showed that only the ether metabolite had effect on all gram-positive and gram-negative microbial strains. The histopathological examination results showed that the bacterial metabolite has a significant healing effect on Staphylococcus aureus wound. The antimicrobial and antitumor activities can be due to the presence of 1,2-Benzenedicarboxylic acid diisooctyl ester and bis (2-ethylhexyl) phthalate in the metabolite composition (24), which were in line with the present study findings on the anti-cancer effects of the microbial compounds.
The results of MTS and annexin tests showed that the rate of apoptosis in PC-3 cell line increased significantly by increasing the concentration of Bacillus licheniformis supernatant. Considering that cell proliferation and irregular apoptosis are the characteristics of cancer cells, Bacillus licheniformis supernatant can be used to reduce cell proliferation and increase apoptosis for the prevention and treatment of prostate cancer.
We would like to express our gratitude and thanks to all those who helped us during the implementation of this research. It is worth mentioning that this article is the result of a part of the Ph.D thesis of Mr. Ahmadreza Shahniani from the Islamic Azad University, Kazerun Branch. This article was financially supported by the authors.
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
This research resulted from an independent research without receiving any financial support.
Authors declared no conflict of interests.
Received: 2020/08/19 | Accepted: 2020/10/10 | ePublished: 2020/10/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |










