BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1170-en.html
2- Department of Internal Medicine and Clinical Pathology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
.
Diarrhea is a clinical syndrome that causes serious economic losses as it leads to high mortality, weight loss, or even late growth in various animals and even in the human people. It has appeared from enteritis, which is the inflammation of the intestinal mucosa, distinguished by abdominal pain, loose feces, an increase in stool mass, stool frequency, tendency, or stool fluidity that contain 70-95% water which leads to dehydration (1,2). Lamb diarrhea is associated with both infectious and non-infectious factors (1, 2). Salmonella spp. and Escherichia coli is the most common bacterial etiologic agents of lamb diarrhea during the first weeks of life (1, 3). Diarrhea induced by infectious organisms is the most significant cause of morbidity and mortality in ruminant neonatal in the world and it can be caused by numerous pathogens including viruses, protozoa, and bacteria (1, 3). Currently, there is some medication approved for the treatment of bacterial pathogens of diarrhea in the animal. Chloramphenicol, gentamicin and Enrofloxacin are used to treat bacterial-induced diarrhea in ruminants’ neonates. These agents have several side effects, including liver and kidney failure including toxic nephrosis and hemorrhage, as well as removing the natural microflora of the digestive tract when used orally (4). Nowadays, due to the creation of microbial resistance to antibiotics, the use of other safe sources such as derived agents from plants and their compounds have been proposed as an alternative to synthetic antibiotics. Recently the results of many studies have shown that some plants interestingly can inhibit the growth of the microorganisms (5). Malva sylvestris L. an annual plant known as mallow is a genus belonging to the family Malvaceae with lobed leaves and purple flowers that bloom in late spring (6). M. sylvestris L. can be used as food antimicrobial agents due to their potent activity against both gram-positive and gram-negative bacteria (7, 8). Therefore, the present study was conducted to evaluate the antibacterial properties of M. sylvestris L. against Salmonella enterica and E. coli isolated from diarrheic lambs.
Plant Extraction
During summer 2018 M. sylvestris was obtained from the local market in Urmia, Iran, then the sample was authenticated by the Pharmacognosy department. Leaves were air-dried at room temperature and grounded into powder by a hammer mill. To prepare the extract, 200 g of powder was macerated in 70% ethanol (1:10 ratio) in a sealed container and was shaken for 24 hr in a darkroom. The extract was filtered through Whatman No 41 filter paper and concentrated under vacuum at 40°C using a rotary machine, and collected powder was stored at −80°C (9).
Isolation and Identification of Bacteria
In this study, 100 stool specimens collected from diarrheic Lambs were refereed to veterinary teaching hospital and clinic of Urmia University during the six months in winter and spring 2018. The strains of bacteria were identified by the use of biochemical profiles according to the directions of the manual of clinical microbiology. The assayed microorganisms used in this study were as followed: 1) Regional clinical isolates: S. enterica (n=10), E. coli (n=10). 2) And Reference strains: S. enterica PTCC 1709-CIP104115, E. coli PTCC1270. The standard strains were taken from Microbiology Tabriz and the Hospital of Department of Urmia University (10).
Antibacterial Activity Assay
The antibacterial activity of the extract was assessed using agar disk-diffusion, micro broth dilution (11). The minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC) were evaluated. Bacterial suspensions equivalent to a 0.5 McFarland turbidity were made in sterile normal saline solution from clinical and reference isolates a sterile swab dipped into the inoculum tube containing bacterial suspensions and then was cultured on the Muller-Hinton agar (Merck®, Germany). Sterile filter paper discs (6 mm in diameter) were impregnated M. sylvestris extract 10 µl (75 mg mL-1) for 10–15 min and left to dry completely for 20–25 min, then precisely placed on the surface of previously inoculated cultures. Chloramphenicol (30µg) antibiotic discs (Merck®, Germany) were used as positive control and sterile diluent (0.1% peptone water) was negative control for comparison of inhibition zone with the sample. Plates were incubated at 37°C for 24h until the obvious growth of bacteria was evident in control plates. Apparent inhibition zones around discs were measured in three directions and averaged. The antibacterial activity was displayed as the diameter of the inhibition zone produced by extract against test bacteria (12).
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
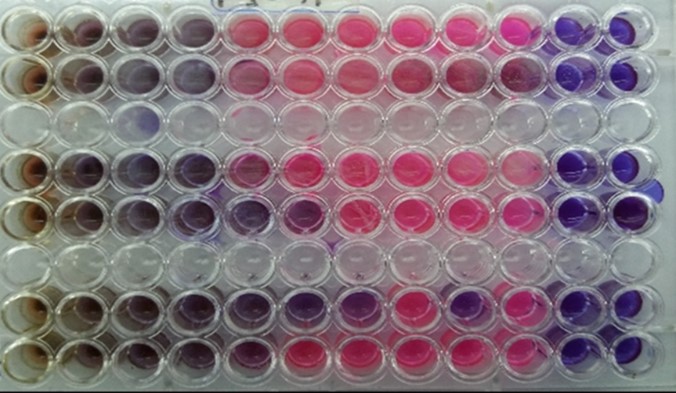
The broth microdilution method was conducted to determine the MIC and MBC of the extract revealed by the agar diffusion assay (13). Briefly, MIC and MBC were tested in the microplate reader, using sterile 96 wells plates. Each well was loaded with a total volume of 100 µl containing Mueller-Hinton broth (MHB). Different concentrations of each extract 100, 50, 25, 12.5, 6.25 and 3.12 mg mL-1 was provided by serial dilution (dilution by one-half) in MHB. 100 µl of inoculums contains approximately 5×105 CFU/mL of test bacteria were added to each well. Negative controls contained a non-inoculated medium with extract samples and positive controls wells were prepared with inoculated culture medium with no extracts [13]. Resazurin powder (11) (Sigma-Aldrich) was diluted in distilled water to a final concentration of 1 mg/ml and 10 µL was added to all wells (13). Microplates were incubated at 37°C for 24 hr. The MIC was determined by observing the lowest concentration of extract which would inhibit the apparent growth of bacteria. For determination of minimum bactericidal concentrations (MBC), 20 µL of the suspension of well before MIC of the extract were cultured on BHI agar using the spread plate technique. After 24 hours of incubation at 37°C, the MBC was evaluated by calculating the number of bacterial colonies (13).
The antimicrobial characteristics of the M. sylvestris against clinically isolates of S. enterica and E. coli were determined and presented as MICs and MBCs values. M. sylvestris extract showed the highest effect against clinical isolate of E. coli (MIC: 11.56±0.0 mg mL-1 and MBC: 21.25±0.0 mg mL-1). Details presented in Tables 1 and 2. Bacterial enteritis in lambs is the most common and important disease that can cause severe economic loss in the sheep production industry. Lambs are at greatest risk of diarrhea during the first month of the neonatal period, although the incidence of diarrhea declines with age (14). Enteropathogens have a high level of resistance to commonly used antibiotics therefore resistance to new drugs develops quite fast. Diarrheal diseases are greatly more common among lambs group and the search for new agents with antibacterial activity against enteropathogens has a public health priority (1, 2). New antimicrobials derived from medicinal plants resulting in recovery without side effects. This is very important since a major problem with antibiotics is a common failure to achieve successful treatment, which in turn favors the selection of resistant bacterial strains. With the focus of research on the undesired effects of chemical drugs, the use of herbal medicines has been re-considered (16). Most of the plant extracts have antimicrobial activity, which is mainly associated with their phenolic compounds. The higher amount of phenolic material in the plant extracts appears more antimicrobial activity (16). In a study by 17. Mohajerfar that was done on 10 different species of bacteria, it is shown that the methanol extract of M. sylvestris had an antibacterial effect on B. pumilus (17). The results of another study showed that the extract of M. rotundifolio had a strong inhibiting effect on S. aureus, P. aeruginosa, B. subtilis, K. pneumoniae, and E. coli (18). It was revealed that methanolic extracts of the flower and leaves of M. sylvestris show high antimicrobial activity against S. aureus, S. agalactiae, and E. faecalis (22). Dost-Mohamadi et al. (2012) mentioned that the ethanolic extracts of Malva neglecta with nanosilver particles had an inhibitory effect on S. aureus and S. typhimurium which were consistent with our findings (19). Elvin-lewis et al. (2009) demonstrated the antibacterial effect of M. neglecta on the Nocardia strain (15). The result of this study confirmed that the M. sylvestris has an antibacterial effect against Salmonella enterica and E.coli. According to the results presented here and from previous research, M. sylvestris potentially has a wide-spectrum antimicrobial effect. Studies are needed to characterize the accurate and precise mechanisms of M. sylvestris ingredients and to study its toxicity to further define the potential therapeutic benefit of it or the risk that accompanies oral administration of this plant extract respectively.
Table 1. MICs and MBCs of M. sylvestris extract against standard and clinically isolated bacteria
| Bacteria | MIC (mg mL-1) | MBC (mg mL-1) |
| S. enteric (1709-CIP104115) | 50±0.0 | 100±0.0 |
| S. enteric (clinical isolate) | 42.5±0.0 | 80±0.0 |
| E. coli (PTCC -1270) | 50±0.0 | 100±0.0 |
| E. coli (clinical isolate) | 11.56±0.0 | 21.25±0.0 |
| Bacteria | M. sylvestris (75 mg mL-1) |
Chloramphenicol (0.03 mg/disc) |
| S. enteric (1709-CIP104115) | 10.2 | 13.9 |
| S. enteric (clinical isolate) | 15 | 17 |
| E. coli (PTCC -1270) | 13.3 | 16 |
| E. coli (clinical isolate) | 16 | 20 |
The results of this study indicate that M. sylvestris has antibacterial properties. In particular, M. sylvestis can be considered as a suitable substitute for synthetic antibiotics to treat the bacterial diarrheic lambs as a cheap and available source. Certainly, more studies are needed to consider the mechanisms of antibacterial effects of M. sylvestris and its compounds more accurately. Therefore, taking into account the detrimental effects of antibiotics, the use of medicinal plants-based drugs can be a new horizon for controlling and controlling diarrhea pathogens especially bacteria.
We thank Hospital of Urmia and Microbiology Department of Urmia University
All authors disclose any financial and the authors declare that there are not any potential conflicts of interest.
Received: 2020/06/18 | Accepted: 2020/11/19 | ePublished: 2021/01/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |