BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijmm.ir/article-1-1149-en.html

 , Rasool Madani
, Rasool Madani 
 2, Parviz Pakzad1
2, Parviz Pakzad1 
 , Fariba Golchinfar3
, Fariba Golchinfar3 
 , Tara Emami3
, Tara Emami3 

2- Department of Proteomics and Biochemistry, Razi vaccine and serum research institute, Agricultural research education and extension organization (AREEO), Karaj, Iran , madanirasool@gmail.com
3- Department of Proteomics and Biochemistry, Razi vaccine and serum research institute, Agricultural research education and extension organization (AREEO), Karaj, Iran
.
IInfluenza is a globally important respiratory viral infection in humans that is easily transmitted from person to person with high mortality and morbidity rates and profound economic impact (1). Almost every year, there are epidemics of 3-5 million cases of severe influenza worldwide, that there is an estimate of 250,000-500,000 deaths annually (2).
There are four types of influenza viruses: A, B, C, and D (3). Avian influenza (AI) is caused by the influenza A virus, which belongs to the Orthomyxoviridae family, genus Influenza virus A. It rapidly infects poultry when it breaks out (4). Many species of birds are susceptible to avian influenza virus (AIV). The widespread epidemic of AI in domestic birds increases the likelihood of mutational events and genetic reassortment, threatening a future pandemic of AI. Adequate surveillance, including serologic detection of antibodies to AIV in birds, as well as other susceptible species, is of great importance in preventing and controlling AI (5).
AIV is an enveloped virus (6). There are three viral proteins on the membrane: the hemagglutinin (HA), neuraminidase (NA), and the M2 proton channel (7,8). AIV bearing 16 antigenic subtypes of HA and 9 antigenic subtypes of NA, which have been isolated from waterfowl and shorebirds. Each virion contains eight segments of negative-sense viral RNA (vRNA) assembled as individual viral ribonucleoproteins. Each viral ribonucleoprotein contains the viral polymerase, which consists of PA, PB1, and PB2 subunits, the nucleoprotein (NP), and a single strand of vRNA (9,10).
NP plays a central role in viral replication. As a structural protein with no intrinsic enzymatic activity, it is the most abundant viral protein in infected cells. NP is a critical component of the vRNA complex, and the recognized functions of NP include but are not limited to, organization of RNA packing, nuclear trafficking (11,12) and vRNA transcription and replication (10).
A filtered and purified influenza A vaccine for humans has been developed, and many countries have stockpiled it to allow a quick administration to the population in the event of an AI pandemic. Researchers reported the discovery of an antibody effective against all types of the influenza A virus (13,14).
Immunization with the whole virus induces mainly humoral immune responses to the viral surface glycoproteins, HA, and NA. However, this immunity is strain-specific and gives little protection against drifted variants of the virus (15). In contrast, immunization with NP induces a wide spectrum of immune responses (16,17). This immunity is cross-reactive and recognizes conserved epitopes within viral proteins (18,19), affording protection against different subtypes of influenza A virus, which contains multiple immuno-dominant epitopes (20). Most probably, broadly protective influenza vaccines are based on conserved proteins, such as NP. NP is a vaccine target of interest as it has been shown to induce cross-reactive antibody and T cell responses (20). In this study immunogenicity of AIV nucleoprotein was evaluated. Therefore, by identifying the role of NP immunity, it can be used for diagnostic kits.
Viruses
The wild-type virus A/Chicken/Iran/259/2014 (N9H2) was obtained from the poultry vaccine research department of the Razi vaccine and serum research institute (RVSRI).
Determination of Protein Concentration
To perform electrophoresis and purification of nucleoprotein, the protein concentration of the samples was determined by Lowry method. Protein concentration was measured by using bovine serum albumin (BSA) as standard (21).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
Electrophoresis on 10% polyacrylamide gel was performed according to the method of Laemmli (22). Both SDS-PAGE and Native-PAGE electrophoresis were used and the differences in gel bands in the two methods were evaluated. Native-PAGE gel electrophoresis was performed in a 10% resolving gel (23). Gel and reservoir buffer did not contain SDS. The sample was prepared in sample buffer without β-mercaptoethanol, SDS, or heating.
Purification of the Virus Nucleoprotein
Purification of the virus nucleoprotein was performed by the electroelution method (24). In this procedure, an SDS-polyacrylamide gel containing electrophoresed proteins was stained with Coomassie blue, the gel band of interest was excised, and the stained protein was electrophoretically eluted from the minced gel. An elution flow of 10 to 8 mA/tube was performed for 3-5 hours. After the removal of most of the salt and SDS by dialysis, the protein was concentrated with polyethylene glycol (PEG) and then analyzed.
Mouse Immunization
Immunogenicity studies in mice were conducted to evaluate the immunogenic potential of the H9N2 virus and to determine the minimal effective dose that generates protective correlates of immunity. Two sets of immunization experiments were performed using prime/boost strategy. In the first immunization, experimental groups of mice were vaccinated subcutaneously with 40 and 35 μg of NP antigen (mice A and B) and boosted twice at three-week intervals. Also, other mice were immunized subcutaneously with 27 μg of the H9N2 virus. Control mice received 70 μL of PBS subcutaneously. Blood samples were collected seven days after the last injection from the corner of the eye. Mouse sera were collected; the antibody titers were measured by the Enzyme-Linked Immunosorbent Assay (ELISA).
Enzyme-Linked Immunosorbent Assay (ELISA)
The sera of immunized mice with NP were evaluated by the ELISA method to obtain the produced antibody titers. The ELISA protocol was performed as described previously (4). For this purpose, a checkerboard was designed using NP as an antigen and different dilutions of sera. At each stage, one negative and one positive serum were considered together as control samples. One negative serum was tested in ELISA to establish endpoint cutoff. The antibody titer for unknown sera was calculated as the reciprocal of the highest dilution of the sera that gave a 450 nm greater than the mean 450 nm of the one negative serum.
Double-Immunodiffusion Assay (Ouchterlony)
Double immunodiffusion is a simple gel-based assay for detecting antigen-specific antibodies. Agar gels prepared in phosphate buffer pH:7 and poured onto plates. Small wells were punched 0.5 to 0.75 cm apart in the analytical gel. Antigen sample (NP) was poured into the central well and serum samples were poured into other wells with different dilutions and allowed to diffuse into the gel for 6 to 48 hr. As antibody and antigen form diffusion gradients that cross each other, a line of immune-precipitation may form between the wells, indicating the presence of specific antibodies. The gel was then stained and destained until precipitin lines are maximally visible (25,26).
Western Blot
After electrophoresis, the gel was cut into two parts. One part of the gel was stained and the other part transferred to nitrocellulose membrane for western blot detection (27). The second part of the gel was placed on a plate containing transfer buffer, at 4°C for 30 min. Then the gel was applied to the nitrocellulose membrane and Western blotting was done for 30 min at 10 V. Nitrocellulose membrane was withdrawn and placed in a blocking buffer (BSA 3% in PBS) for 90 min. The nitrocellulose membrane was washed three times with washing buffer (PBST). After washing, the antibody (immune sera of injected mouse; 1:15 dilution) was poured on the nitrocellulose membrane and incubated overnight at 4°C. The fixed plate was washed four times with PBS-Tween (PBST) washing buffer and then it was incubated with HRP-IgG (goat anti-mouse immunoglobulin G antibodies conjugated with horseradish peroxidase) diluted at 1:500 for 1 hr. The plate was washed five times with washing buffer. After this step, the membrane was incubated in the precipitant chloronaphthol substrate (18mg chloronaphthol, 6mL methanol and, 9 μL hydrogen peroxide in 24 mL PBS). After 15 min of color development, the nitrocellulose membrane was photographed.
The molecular weights of proteins from the H9N2 ranged from 30 to 140 kDa in SDS-PAGE and Native-PAGE (Figure 1). The NP contained a single band with a molecular weight 60 kDa (Figures 1 and 2).
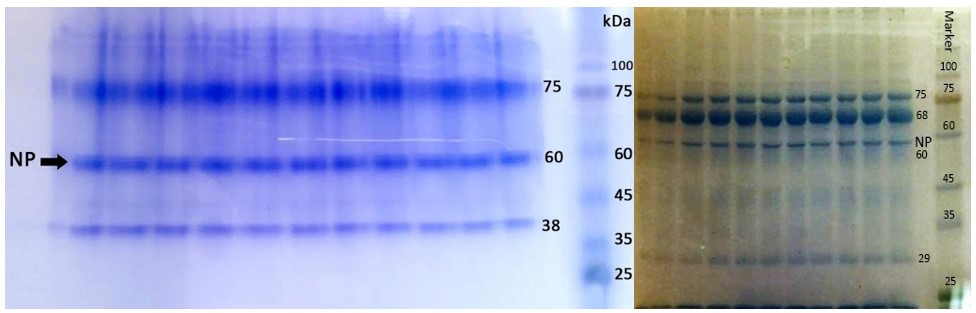
Figure 1. SDS-PAGE (left) and Native-PAGE (right) of N9H2 and its proteins (coomassie blue staining)

Figure 2. SDS-PAGE of N9H2 and its proteins (silver nitrate staining)
Ouchterlony showed that in serum dilution of 1:2, and 1:4, the sediment lines were formed between the serum and the NP antigen (Figure 3).
By ELISA, the optimal concentration of NP antigen was about 1 µg/mL at a 1:20 dilution. Thus, the NP-ELISA enables rapid serological diagnosis and is suited for influenza A antibody screening (Table 1).

Figure 3. Ouchterlony of NP
Table 1. Absorbance of the samples
| NP Ag 2 µg/ml |
NP Ag 1 µg/ml |
NP Ag 0.5 µg/ml |
NP Ag 0.25 µg/ml |
Serum of mice | Dilution of sera |
| 0.968 | 0.991 | 0.799 | 0.532 | Mice 1 | 1:20 |
| 1.000 | 0.932 | 0.854 | 0.630 | Mice 2 | |
| 2.161 | 2.117 | 1.813 | 1.624 | Control + | |
| 0.471 | 0.322 | 0.308 | 0.298 | Control - | |
| 0.718 | 0.736 | 0.680 | 0.498 | Mice 1 | 1:40 |
| 0.593 | 0.591 | 0.636 | 0.574 | Mice 2 | |
| 1.217 | 1.183 | 1.139 | 1.010 | Control + | |
| 0.425 | 0.311 | 0.295 | 0.251 | Control - | |
| 0.366 | 0.470 | 0.407 | 0.358 | Mice 1 | 1:80 |
| 0.337 | 0.588 | 0.495 | 0.399 | Mice 2 | |
| 0.988 | 0.900 | 0.856 | 0.987 | Control + | |
| 0.361 | 0.229 | 0.207 | 0.200 | Control - |
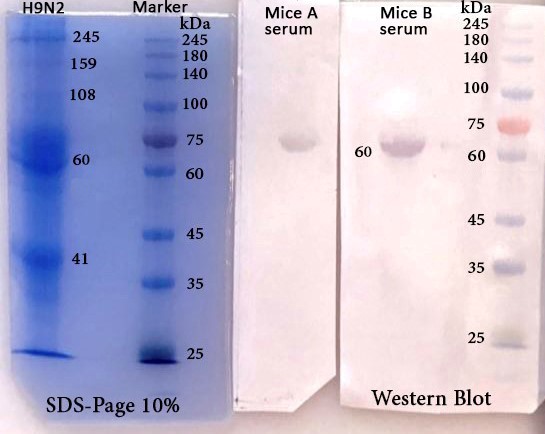
Figure 4. Western blot of NP
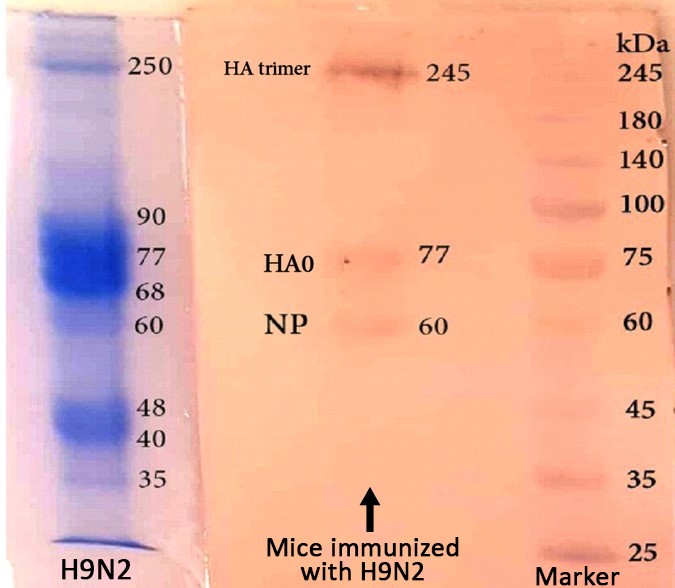
Figure 5. Western blot of N9H2
Influenza viruses infect many animal species in addition to humans that infects more than 5 million people each year in the world. Influenza epidemics are estimated to cause approximately 500,000 deaths per year worldwide. In this case, AI is one of the most important viral diseases that is a syndrome of poultry caused by type A influenza virus (2,28).
The accurate and prompt diagnosis of AIV infection in birds is a critical component of surveillance and disease control. Given the prevalence of AI subtype H9N2 as an infectious agent in the poultry industry, special attention has been always directed toward the development of vaccine production against this infection. Vaccination would be the best strategy to control AI with pandemic strains and is used in different countries, such as Iran, China, Pakistan, and Korea (29).
But, it has to be noted that according to the antigenic shift in influenza viruses, prevention by vaccination has always been a problem. The world health organization (WHO) collects information on the prevalence of influenza worldwide every year to introduce a viral strain for vaccine production next year. Inactivated viral vaccines cannot induce local antibodies or stimulate cell-mediated immune responses. Because of culturing selected strains for vaccination in embryonated chicken eggs and the harvest of the virus from allantoic fluid, the presence of egg protein antigens also has side effects for people with a history of allergy to the egg protein. In contrast, live attenuated viral vaccines can produce neutralizing antibodies and provide cell-mediated immunity, but this vaccine also has limitations, including the need for two doses of vaccine to induce optimal immune responses. This vaccine is not prescribed in children under the age of two years and adults over 51 years of age due to the potential risk of the presence of a live virus. Also, these vaccines are produced during difficult and time-consuming processes, similar to inactivated vaccines (30,31).
Serological detection of AI is still the most important detection test. The standard method for serological detection of AIV infection [such as haemagglutination and neuraminidase inhibition (HI and NI) tests], has been shown to be less sensitive for the detection of antibodies induced by AIVs. Also, the HI assay is limited to various HA subtypes, and biosecurity is a concern when working with a live virus (32). Thus, it is important to develop serological detection assays for specific antibodies to all subtypes of AIV.
Molecular methods, such as polymerase chain reaction (PCR) (33) and ELISA (34), have also been used for AIV diagnosis. Although these methods are rapid, sensitive, and specific, but the test results often need to be confirmed by additional tests, such as Western blot. In the present study, attempts were made to develop Western blot for detecting antibodies against NP of AI viruses. With the high degree of conservation and group-specificity of NP, the western blot with NP developed in this study could detect the H9N2 subtype of AIV, and we believe that it can detect AI antibodies to all subtypes.
Concerns about the pandemic caused by the rearrangement of human viruses and highly acute avian viruses as well as the unexpected pandemics have led the researchers to detect AI using new serological methods based on conserved viral proteins (such as NP). The immune response generated by immunization with conserved proteins is expected to act as the first line of defense and protect society against acute influenza by launching a widespread immunization pending the preparation of a strain-specific vaccine (16).
Recent studies have shown that influenza virus proteins, including NP, can protect animals against different types of influenza by stimulating appropriate immune responses. These findings have set the hope for better detection and development of this type of vaccine against seasonal and pandemic influenza in the near future (35). The results of this study are consistent with the present study so that the western Blot test using NP and its immunodominant test revealed that NP has the potential to stimulate the immune system and can be used to manufacture diagnostic kits.
In addition to the western blot, ELISA in this study is based on NP, which is highly conserved among AI and can be used as a candidate diagnostic antigen because of its group and type specificity. Accordingly, in this work, we developed an indirect ELISA adapted to detect AIV type-specific antibody based on the NP antigen. The NP antigen successfully blotted AI-positive sera. These results indicate that NP antigen had immunological specificity and the NP-ELISA was successful for diagnosis.
This study was planned because little information is available about the efficacy of AIVs NP. The NP is one of the most important internal proteins of the influenza virus, which is one of the specific antigens of the type and is used to detect all kinds of influenza viruses. Studies show that internal proteins, such as NP, are one of the most important determinants of the host spectrum of influenza A viruses. Therefore, it is necessary to examine the internal proteins in AIVs in order to determine the changes made in the world, including Iran.
The present study utilized electrophoresis to detect protein bands from isolated influenza virus. According to the results, the nucleoprotein band had a molecular weight of 60kDa. This molecular weight for the nucleoprotein obtained from the present study is in line with previous studies, in which the molecular weight of nucleoprotein was reported to be about 56 to 61kDa (36,37).
The rapid diagnosis of any avian disease is not as important as acute AI. Different methods are available for the study of AI. Western Blot is one of the most important methods. Several reports have recently been made on the immunization of AI using techniques such as western blot, which indicates the importance of applying these methods (38). Accordingly, the immunodominancy of viral proteins and their immunogenicity was investigated and poultry serums for the presence of AIVs using nucleoprotein was analyzed for use in the development of diagnostic kits. The presence of AIV subtype H9N2 in the examined samples indicates that the poultry farming of the country faces this subtype despite heavy losses in some aviculture units.
According to the results of electrophoresis and western blot, the immunodominant proteins of H9N2 influenza virus were detected. It can be stated that the detection of influenza virus and its virulence determination are of the important priorities of prevention and control of the disease. In general, the efficacy of the western blot technique indicates that this method is effective for the detection of influenza.
In this project, the immunogenic proteins were obtained by receiving inactivated virus and immunedominant NP, producing positive and negative chicken serums using immunization and virus analysis and achieving the best concentration of NP purified from H9N2 virus as an antigen, which can be used to design and product a kit using the NP. The kit designed with these specifications has a higher diagnostic value and is more cost effective than commercially available kits.
The results of this study highlight the potential of NPs-based AIV antigens for promoting the induction of both systemic and mucosal immune responses against respiratory pathogens. The Western blot with NP provides an alternative, inexpensive and rapid serological diagnostic tool and is suitable for influenza A antibody screening, especially in species that harbour several influenza subtypes. It can be used in the diagnosis and the serological epidemiological investigation of AI, especially in developing and undeveloped countries.
The authors thank all those who helped them writing this article.
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
This research resulted from an independent research without receiving any financial support.
Authors declared no conflict of interests.
Received: 2020/05/25 | Accepted: 2020/09/20 | ePublished: 2020/11/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





